Crystallization of domains involved in self-assembly of the S-layer protein SbsC.
IF 0.9
4区 生物学
Acta Crystallographica Section F-structural Biology and Crystallization Communications
Pub Date : 2012-12-01
Epub Date: 2012-11-14
DOI:10.1107/S1744309112042650
引用次数: 8
Abstract
The Gram-positive bacterium Geobacillus stearothermophilus ATCC 12980 is completely covered with a two-dimensional crystalline monolayer composed of the S-layer protein SbsC. In order to complete the structure of the full-length protein, additional soluble constructs containing the crucial domains for self-assembly have been successfully cloned, expressed and purified. Crystals obtained from three different recombinant constructs yielded diffraction to 3.4, 2.8 and 1.5 Å resolution. Native data have been collected.
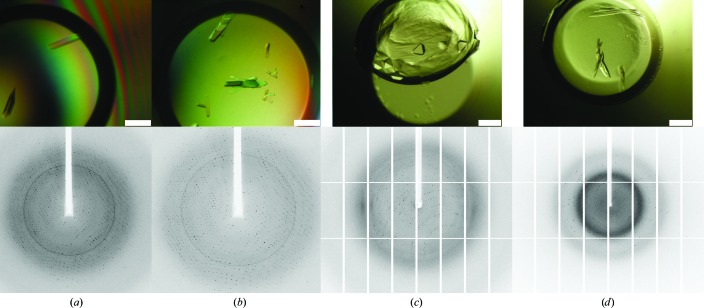
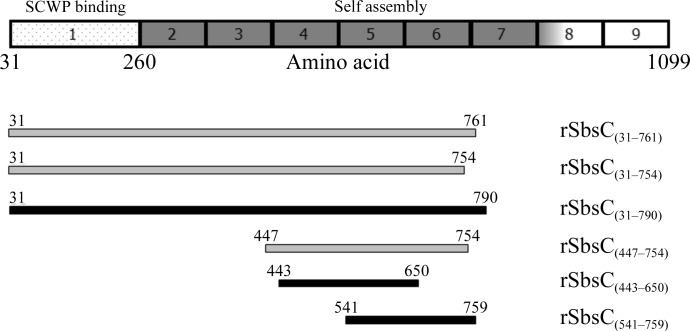
s层蛋白SbsC自组装结构域的结晶。
革兰氏阳性细菌嗜热脂肪土芽孢杆菌ATCC 12980完全被由S层蛋白SbsC组成的二维晶体单层覆盖。为了完成全长蛋白质的结构,已经成功地克隆、表达和纯化了包含自组装关键结构域的额外可溶性构建体。从三种不同的重组构建体获得的晶体产生3.4、2.8和1.5的衍射 Å分辨率。已经收集了本机数据。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
自引率
0.00%
发文量
0
审稿时长
2-4 weeks
期刊介绍:
Acta Crystallographica Section F is a rapid structural biology communications journal.
Articles on any aspect of structural biology, including structures determined using high-throughput methods or from iterative studies such as those used in the pharmaceutical industry, are welcomed by the journal.
The journal offers the option of open access, and all communications benefit from unlimited free use of colour illustrations and no page charges. Authors are encouraged to submit multimedia content for publication with their articles.
Acta Cryst. F has a dedicated online tool called publBio that is designed to make the preparation and submission of articles easier for authors.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


