Structural Chemistry of Human SET Domain Protein Methyltransferases.
Current chemical genomics
Pub Date : 2011-01-01
Epub Date: 2011-08-22
DOI:10.2174/1875397301005010085
引用次数: 0
Abstract
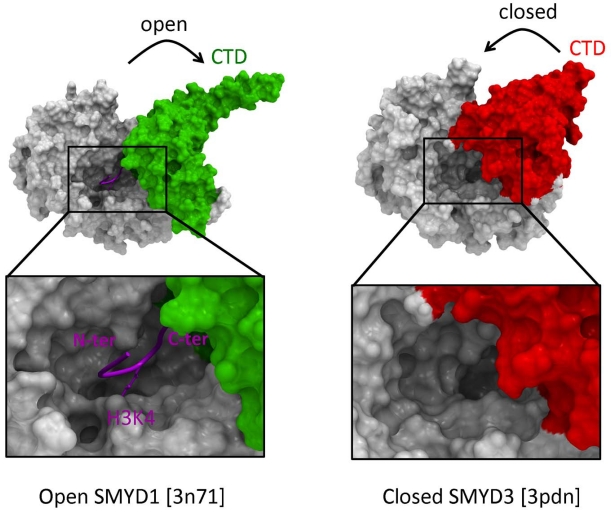
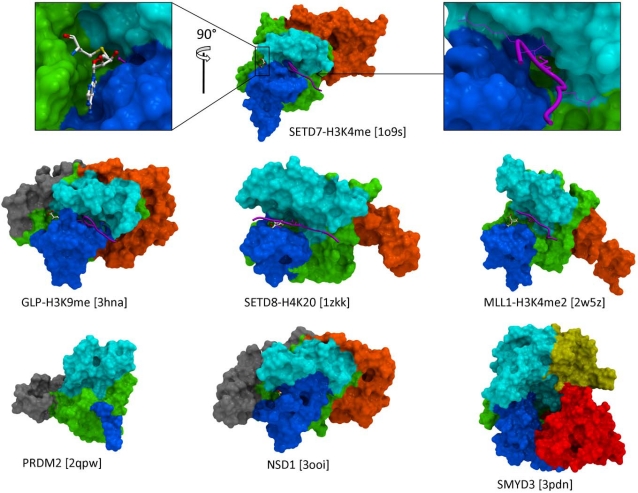
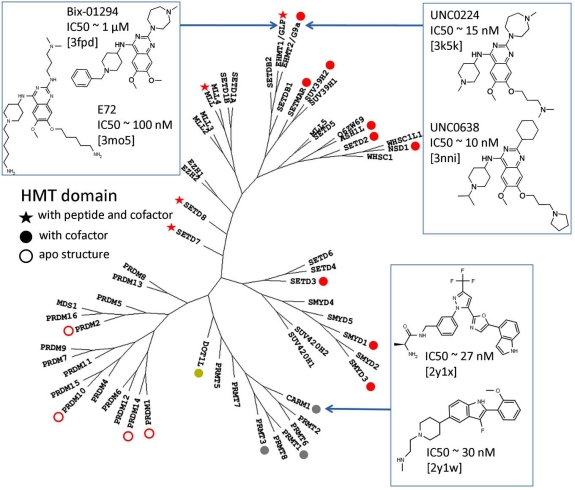
There are about fifty SET domain protein methyltransferases (PMTs) in the human genome, that transfer a methyl group from S-adenosyl-L-methionine (SAM) to substrate lysines on histone tails or other peptides. A number of structures in complex with cofactor, substrate, or inhibitors revealed the mechanisms of substrate recognition, methylation state specificity, and chemical inhibition. Based on these structures, we review the structural chemistry of SET domain PMTs, and propose general concepts towards the development of selective inhibitors.
人类 SET 结构域蛋白甲基转移酶的结构化学。
人类基因组中约有 50 个 SET 结构域蛋白甲基转移酶(PMT),可将 S-腺苷-L-蛋氨酸(SAM)上的甲基转移到组蛋白尾部或其他肽上的底物赖氨酸上。一些与辅助因子、底物或抑制剂复合的结构揭示了底物识别、甲基化状态特异性和化学抑制的机制。基于这些结构,我们回顾了 SET 结构域 PMT 的结构化学,并提出了开发选择性抑制剂的一般概念。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


