The effects of anti-insulin antibodies and cross-reactivity with human recombinant insulin analogues in the E170 insulin immunometric assay.
引用次数: 17
Abstract
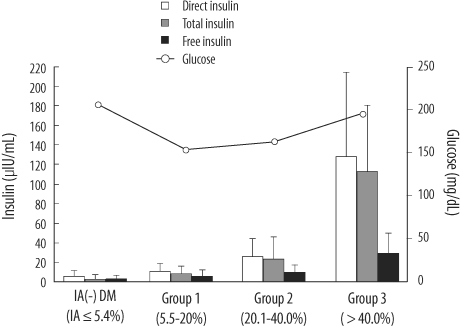
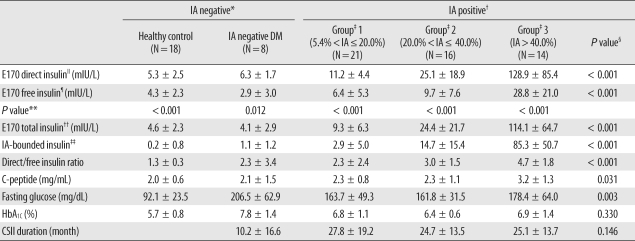
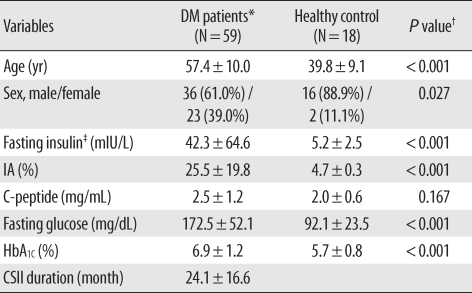
Background Insulin assays are affected by varying degrees of interference from anti-insulin antibodies (IAs) and by cross-reactivity with recombinant insulin analogues. We evaluated the usefulness of the E170 insulin assay by assessing IA effects and cross-reactivity with 2 analogues. Methods Sera were obtained from 59 type 2 diabetes patients receiving continuous subcutaneous insulin infusion and 18 healthy controls. Insulin levels were determined using an E170 analyzer. To investigate the effects of IAs, we performed IA radioimmunoassays, and analyzed the differences between directly measured insulin (direct insulin) and polyethylene glycol (PEG)-treated insulins (free, IA-unbound; total, IA-bound and unbound insulin). We performed in-vitro cross-reactivity tests with insulin aspart and insulin glulisine. Results In IA-positive patients, E170 free insulin levels measured using the E170 analyzer were significantly lower than the direct insulin levels. The mean value of the direct/free insulin ratio and IA-bound insulin, which were calculated as the difference between total and free insulin, increased significantly as endogenous IA levels increased. The E170 insulin assay showed low cross-reactivities with both analogues (< 0.7%). Conclusions IAs interfered with E170 insulin assay, and the extent of interference correlated with the IA levels, which may be attributable to the increase in IA-bound insulin, and not to an error in the assay. The E170 insulin assay may measure only endogenous insulin since cross-reactivity is low. Our results suggest that the measurement of free insulin after PEG pre-treatment could be useful for β cell function assessment in diabetic patients undergoing insulin therapy.
抗胰岛素抗体及其与人重组胰岛素类似物在E170胰岛素免疫测定中的交叉反应性的影响。
背景:胰岛素测定受到抗胰岛素抗体(IAs)的不同程度干扰以及重组胰岛素类似物的交叉反应性的影响。我们通过评估IA效应和与2种类似物的交叉反应性来评估E170胰岛素检测的有效性。方法:采集59例持续皮下注射胰岛素的2型糖尿病患者和18例健康对照者的血清。胰岛素水平用E170分析仪测定。为了研究IAs的作用,我们进行了IA放射免疫测定,并分析了直接测量的胰岛素(直接胰岛素)和聚乙二醇(PEG)处理的胰岛素(游离,IA未结合;总胰岛素、ia结合胰岛素和未结合胰岛素)。我们进行了胰岛素分离和胰岛素葡氨酸的体外交叉反应性试验。结果:在ia阳性患者中,使用E170分析仪测量的游离胰岛素水平明显低于直接胰岛素水平。直接/游离胰岛素比值和IA结合胰岛素的平均值(总胰岛素与游离胰岛素之差)随着内源性IA水平的升高而显著升高。E170胰岛素试验与两种类似物的交叉反应性较低(< 0.7%)。结论:IAs干扰E170胰岛素测定,干扰程度与IA水平相关,这可能是由于IA结合胰岛素的增加,而不是由于测定错误。由于交叉反应性低,E170胰岛素测定法只能测量内源性胰岛素。我们的研究结果表明,PEG预处理后游离胰岛素的测量可能对接受胰岛素治疗的糖尿病患者的β细胞功能评估有用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


