Homologation of Electron-Rich Benzyl Bromide Derivatives via Diazo C–C Bond Insertion
IF 14.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 3
Abstract
The ability to manipulate C–C bonds for selective chemical transformations is challenging and represents a growing area of research. Here, we report a formal insertion of diazo compounds into the “unactivated” C–C bond of benzyl bromide derivatives catalyzed by a simple Lewis acid. The homologation reaction proceeds via the intermediacy of a phenonium ion, and the products contain benzylic quaternary centers and an alkyl bromide amenable to further derivatization. Computational analysis provides critical insight into the reaction mechanism, in particular the key selectivity-determining step.
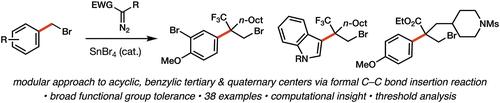
重氮C-C键插入法鉴定富电子苄基溴衍生物
操纵C-C键进行选择性化学转化的能力具有挑战性,并且代表了一个不断增长的研究领域。在这里,我们报道了一个重氮化合物正式插入到苯基溴衍生物的“未活化”的C-C键上,由一个简单的路易斯酸催化。同化反应通过苯离子的中间体进行,产物含有苯基季中心和可进一步衍生化的烷基溴。计算分析提供了关键的洞察反应机理,特别是关键的选择决定步骤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


