Non-faradaic electrochemical impedimetric profiling of procalcitonin and C-reactive protein as a dual marker biosensor for early sepsis detection
Abstract
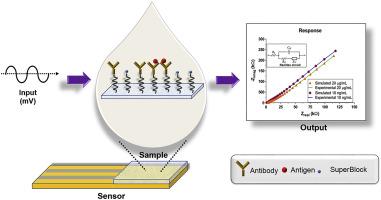
In this work, we demonstrate a robust, dual marker, biosensing strategy for specific and sensitive electrochemical response of Procalcitonin and C-reactive protein in complex body fluids such as human serum and whole blood for the detection of sepsis. Enhanced sensitivity is achieved by leveraging the physicochemical properties of zinc oxide at the electrode-solution interface. Characterization techniques such as SEM, EDAX, AFM, FTIR and fluorescence microscopy were performed to ensure a suitable biosensing surface. The characteristic biomolecular interactions between the target analyte and specific capture probe is quantified through unique frequency signatures using non-faradaic electrochemical impedance spectroscopy (EIS). The developed biosensor demonstrated a detection limit of 0.10 ng mL−1 for PCT in human serum and whole blood with an R2 of 0.99 and 0.98 respectively. CRP demonstrated a detection limit of 0.10 μg mL−1 in human serum and whole blood with an R2 of 0.90 and 0.98 respectively. Cross-reactivity analysis demonstrated robust selectivity to PCT and CRP with negligible interaction to non-specific biomolecules. The novel aspect of this technology is the ability to fine-tune individual biomarkers response owing to the optimal frequency tuning capability. The developed biosensor requires an ultra-low sample volume of 10 μL without the need for sample dilution for rapid analysis. We envision the developed dual marker biosensor to be useful as a sepsis-screening device for prognostic monitoring.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


