Catalytic C-H oxidations by nonheme mononuclear Fe(II) complexes of two pentadentate ligands: Evidence for an Fe(IV) oxo intermediate
Abstract
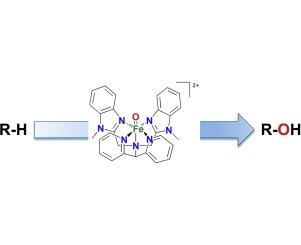
The oxidation reactions of alkanes with hydrogen peroxide and peracids (peracetic acid (PAA) and m-chloroperoxybenzoic acid (mCPBA)) catalysed by two Fe(II) complexes of pentadentate {N5}-donor ligands have been investigated. Kinetic isotope effect experiments and the use of other mechanistic probes have also been performed. While the total yields of oxidized products are similar regardless of oxidant (e.g. 30–39% for oxidation of cyclohexane), the observed alcohol/ketone ratios and kinetic isotope effects differ significantly with different oxidants. Catalytic reactions in H2O2 medium are consistent with the involvement of hydroxyl radicals in the CH bond cleavage step, and resultant low kinetic isotope effect values. On the other hand, catalytic reactions performed using peracid media indicate the involvement of an oxidant different from the hydroxyl radical. For these reactions, the kinetic isotope effect values are relatively high (within a range of 4.2–5.1) and the C3/C2 selectivity parameters in adamantane oxidation are greater than 11, thereby excluding the presence of hydroxyl radicals in the C
H bond cleavage step. A low spin Fe(III)-OOH species has been detected in the H2O2-based catalytic system by UV/Vis, mass spectrometry and EPR spectroscopy, while an Fe(IV)-oxo species is postulated to be the active oxidant in the peracid-based catalytic systems. Computational studies on the C
H oxidation mechanism reveal that while the hydroxyl radical is mainly responsible for the H-atom abstraction in the H2O2-based catalytic system, it is the Fe(IV)-oxo species that abstracts the H-atom from the substrate in the peracid-based catalytic systems, in agreement with the experimental observations.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


