Synergistic Binding of the Halide and Cationic Prime Substrate of l-Lysine 4-Chlorinase, BesD, in Both Ferrous and Ferryl States
IF 3
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
An aliphatic halogenase requires four substrates: 2-oxoglutarate (2OG), halide (Cl- or Br-), the halogenation target ("prime substrate"), and dioxygen. In well-studied cases, the three nongaseous substrates must bind to activate the enzyme's Fe(II) cofactor for efficient capture of O2. Halide, 2OG, and (lastly) O2 all coordinate directly to the cofactor to initiate its conversion to a cis-halo-oxo-iron(IV) (haloferryl) complex, which abstracts hydrogen (H•) from the non-coordinating prime substrate to enable radicaloid carbon-halogen coupling. We dissected the kinetic pathway and thermodynamic linkage in binding of the first three substrates of the l-lysine 4-chlorinase, BesD. After addition of 2OG, subsequent coordination of the halide to the cofactor and binding of cationic l-Lys near the cofactor are associated with strong heterotropic cooperativity. Progression to the haloferryl intermediate upon the addition of O2 does not trap the substrates in the active site and, in fact, markedly diminishes cooperativity between halide and l-Lys. The surprising lability of the BesD•[Fe(IV)=O]•Cl•succinate•l-Lys complex engenders pathways for decay of the haloferryl intermediate that do not result in l-Lys chlorination, especially at low chloride concentrations; one identified pathway involves oxidation of glycerol. The mechanistic data imply (i) that BesD may have evolved from a hydroxylase ancestor either relatively recently or under weak selective pressure for efficient chlorination and (ii) that acquisition of its activity may have involved the emergence of linkage between l-Lys binding and chloride coordination following the loss of the anionic protein-carboxylate iron ligand present in extant hydroxylases.
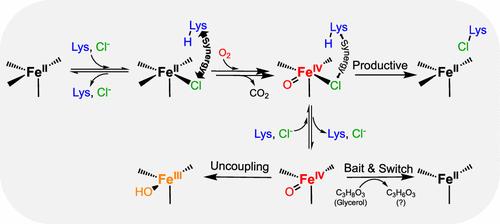
l-赖氨酸4-氯酶(BesD)在亚铁态和铁态的卤化物和阳离子基底物的协同结合
脂肪族卤化酶需要四种底物:2-氧戊二酸酯(2OG)、卤化物(Cl -或Br -)、卤化靶物(“主要底物”)和二氧。在充分研究的情况下,三种非物质底物必须结合以激活酶的Fe(II)辅因子,以有效捕获O2。卤化物,2OG和(最后)O2都直接配位到辅因子上,使其转化为顺式-卤-氧-铁(IV)(卤铁基)配合物,从非配位的主要底物中提取氢(H?),使自由基类碳-卤素偶联。我们剖析了l-赖氨酸4-氯酶前三个底物结合的动力学途径和热力学联系。在加入2OG后,卤化物随后与辅因子的配位以及辅因子附近阳离子l-赖氨酸的结合与强的异向协同性有关。在添加O2后向卤代铁基中间体的进展不会将底物捕获在活性位点,事实上,显著降低了卤化物和l-赖氨酸之间的协同作用。BesD - [Fe(IV)=O] - Cl -琥珀酸盐的惊人稳定性l-赖氨酸复合物产生了不导致l-赖氨酸氯化的卤铁基中间体的衰变途径,特别是在低氯浓度下;一个已确定的途径涉及甘油的氧化。机制数据暗示(i) BesD可能是从一个羟基化酶祖先进化而来的,要么是相对较近的,要么是在有效氯化的弱选择压力下进化而来的;(ii)其活性的获得可能涉及在现有羟化酶中存在的阴离子蛋白-羧酸铁配体丧失后,l-Lys结合和氯离子配位之间的联系的出现。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Biochemistry Biochemistry
生物-生化与分子生物学
CiteScore
5.50
自引率
3.40%
发文量
336
审稿时长
1-2 weeks
期刊介绍:
Biochemistry provides an international forum for publishing exceptional, rigorous, high-impact research across all of biological chemistry. This broad scope includes studies on the chemical, physical, mechanistic, and/or structural basis of biological or cell function, and encompasses the fields of chemical biology, synthetic biology, disease biology, cell biology, nucleic acid biology, neuroscience, structural biology, and biophysics. In addition to traditional Research Articles, Biochemistry also publishes Communications, Viewpoints, and Perspectives, as well as From the Bench articles that report new methods of particular interest to the biological chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


