A new class of cytotoxic agents targets tubulin and disrupts microtubule dynamics
Abstract
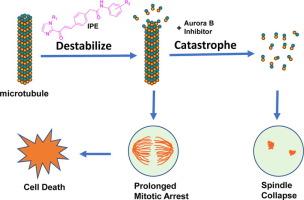
Despite the advances in treatment strategies, cancer is still the second leading cause of death in the USA. A majority of the currently used cancer drugs have limitations in their clinical use due to poor selectivity, toxic side effects and multiple drug resistance, warranting the development of new anticancer drugs of different mechanisms of action. Here we describe the design, synthesis and initial biological evaluation of a new class of antimitotic agents that modulate tubulin polymerization. Structurally, these compounds are chalcone mimics containing a 1-(1H-imidazol-2-yl)ethan-1-one moiety, which was initially introduced to act as a metal-binding group and inhibit histone deacetylase enzymes. Although several analogues selectively inhibited purified HDAC8 with IC50 values in low micromolar range, tissue culture studies suggest that HDAC inhibition is not a major mechanism responsible for cytotoxicity. The compounds demonstrated cell growth inhibition with GI50 values of upper nanomolar to low micromolar potency with significant selectively for cancer over normal cells. Interestingly, several compounds arrested HeLaM cells in mitosis and seem to target tubulin to cause mitotic arrest. For example, when combined with inhibitors of Aurora B kinase, they led to dramatic disassembly of the mitotic spindle. In-vitro tubulin polymerization studies showed that the compounds reduced the rate of polymerization of microtubules during the elongation phase and lowered the amount of polymerized tubulin during the plateau phase. Finally, in silico docking studies identified binding of IPE-7 to the colchicine site with similar affinity as the test compound D64131. These compounds represent a new antimitotic pharmacophore with limited HDAC inhibitory activity.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


