Efficient functionalization of olefins by arylsilanes catalyzed by palladium anionic complexes
IF 5.062
引用次数: 6
Abstract
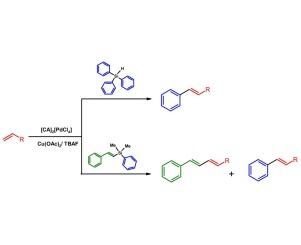
The coupling of organosilanes and different olefins was performed efficiently in the presence of anionic complexes, [CA]2[PdX4] and [CA]2[Pd2X6], where CA = imidazolium or pyridinium cation. The reaction proceeds according to a Pd(II)-mediated pathway, and Cu(OAc)2 acts as the re-oxidant of Pd(0) formed during the catalytic process. High product yields were obtained for differently substituted olefins at 80 °C in 4 h. Styrylsilanes reacted in the same conditions giving unsymmetrical 1,3-dienes.
钯阴离子配合物催化芳基硅烷高效功能化烯烃
在阴离子配合物[CA]2[PdX4]和[CA]2[Pd2X6]的存在下,有机硅烷与不同的烯烃进行了有效的偶联,其中CA =咪唑或吡啶阳离子。反应根据Pd(II)介导的途径进行,Cu(OAc)2作为催化过程中生成的Pd(0)的再氧化剂。不同取代的烯烃在80℃下反应4 h得到了较高的产率。苯乙烯基硅烷在相同条件下反应得到不对称的1,3-二烯。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
自引率
0.00%
发文量
0
审稿时长
2.8 months
期刊介绍:
The Journal of Molecular Catalysis A: Chemical publishes original, rigorous, and scholarly full papers that examine the molecular and atomic aspects of catalytic activation and reaction mechanisms in homogeneous catalysis, heterogeneous catalysis (including supported organometallic catalysis), and computational catalysis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


