Cu(I)-catalyzed one-pot decarboxylation-alkynylation reactions on 1,2,3,4-tetrahydroisoquinolines and one-pot synthesis of triazolyl-1,2,3,4-tetrahydroisoquinolines
IF 5.062
引用次数: 7
Abstract
A facile and efficient method to introduce alkyne groups to the C-1 position of biologically interesting 1,2,3,4-tetrahydroisoquinolines via direct CH-functionalization is reported. Various alkynylated N-substituted 1,2,3,4-tetrahydroisoquinolines could be obtained by using copper(I)-chloride as catalyst, alkynoic acids as alkyne source and t-BuOOH as oxidant, in a one-pot two-step decarboxylation- alkynylation reaction in moderate to high yields. Furthermore, a one-pot protocol of a three-step decarboxylation-alkynylation-1,3-dipolar cycloaddition reaction leading to 1-triazolyl-tetrahydroisoquinolines was developed, a hitherto unknown reaction cascade.
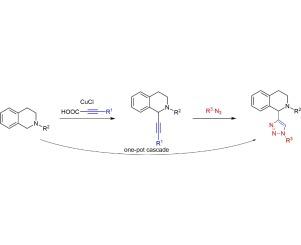
Cu(I)催化1,2,3,4-四氢异喹啉的一锅脱羧-烷基化反应和三唑基-1,2,3,4-四氢异喹啉的一锅合成
报道了一种通过直接ch官能化将1,2,3,4-四氢异喹啉类化合物的C-1位上的炔基引入的简便有效的方法。以氯化铜(I)为催化剂,烷基酸为炔源,叔丁酸为氧化剂,采用一锅两步脱羧-烷基化反应,可制得各种n-取代1,2,3,4-四氢异喹啉,收率中高。此外,我们还建立了一个由脱羧-烷基化-1,3-偶极环加成反应生成1-三唑基-四氢异喹啉这一迄今未知的级联反应的一锅反应方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
自引率
0.00%
发文量
0
审稿时长
2.8 months
期刊介绍:
The Journal of Molecular Catalysis A: Chemical publishes original, rigorous, and scholarly full papers that examine the molecular and atomic aspects of catalytic activation and reaction mechanisms in homogeneous catalysis, heterogeneous catalysis (including supported organometallic catalysis), and computational catalysis.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


