Detection and Elimination of Product Inhibition from the Asymmetric Catalytic Hydrogenation of Enamines
IF 5
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 39
Abstract
The catalytic asymmetric hydrogenation of enamine amides and esters with catalyst Rh-1a, prepared from ferrocenyl based ligand 1a or 1b and [(COD)RhCl]2, has been shown through kinetic studies to suffer from product inhibition. Enamine ester substrates have also been shown to be incompatible with the amine products of the reaction in methanol. In situ protection of the amine products with di-tert-butyl dicarbonate eliminates functional group incompatibility of ester substrates and eliminates product inhibition in the reaction.
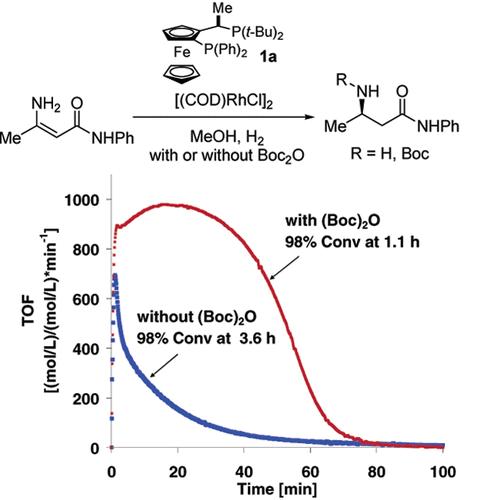
胺类不对称催化加氢产物抑制的检测与消除
以二茂铁基配体1a或1b和[(COD)RhCl]2为原料制备的烯胺酰胺和酯类在Rh-1a催化下的不对称加氢反应受到产物抑制。烯胺酯底物也被证明与甲醇反应的胺产物不相容。用二碳酸二叔丁基原位保护胺产物消除了酯底物的官能团不相容性,消除了反应中的产物抑制作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


