Multimodal deep learning for bone tumor diagnosis with clinical imaging, pathology, and blood biomarkers
IF 3.5
2区 医学
Q2 Medicine
引用次数: 0
Abstract
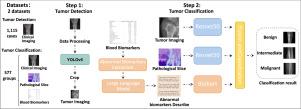
Accurate classification of bone tumors as benign, malignant, or intermediate is crucial for patient treatment decisions. Misclassification may result in overtreatment of benign cases or delayed intervention for aggressive tumors, significantly impacting patient prognosis. However, current methods rely heavily on single-modality imaging analysis, making it difficult to handle variable lesion locations and complex cancer types. To address these limitations, we propose a novel multimodal deep learning framework that integrates clinical images, pathological slices, and blood biomarkers for automated bone tumor detection and three-class classification. The framework operates in two stages: first, a YOLOv5-based detection model localizes tumor regions on clinical images. Next, a classification model utilizes ResNet to extract deep features from both the clinical images and pathological slices, while abnormal blood biomarkers are transformed into descriptive text by a large language model and subsequently encoded into semantic features using BioBERT. Finally, features from all three modalities are integrated via a fusion module to capture complementary information and enable accurate tumor classification. The evaluation was performed using two distinct datasets: a clinical imaging dataset for bone tumor detection, and a separate multi-modal cohort comprising clinical imaging, pathology, and blood biomarkers for tumor classification. The detection model demonstrated strong localization capabilities, achieving a test [email protected] of 0.7925. For the classification task, ablation studies validated the complementary contribution of each modality. Notably, our multimodal fusion approach outperformed unimodal baselines, attaining a macro-average precision of 0.9056, F1-score of 0.8736, and AUC of 0.9759 in tumor classification—outperforming existing models.
基于临床影像、病理和血液生物标志物的骨肿瘤诊断的多模态深度学习
骨肿瘤的准确分类为良性、恶性或中度对患者的治疗决策至关重要。错误分类可能导致良性病例的过度治疗或侵袭性肿瘤的延迟干预,严重影响患者预后。然而,目前的方法严重依赖于单模态成像分析,难以处理不同的病变位置和复杂的癌症类型。为了解决这些限制,我们提出了一种新的多模态深度学习框架,该框架集成了临床图像、病理切片和血液生物标志物,用于自动骨肿瘤检测和三类分类。该框架分为两个阶段:首先,基于yolov5的检测模型在临床图像上定位肿瘤区域。接下来,分类模型利用ResNet从临床图像和病理切片中提取深层特征,而异常血液生物标志物通过大型语言模型转换为描述性文本,随后使用BioBERT编码为语义特征。最后,通过融合模块整合所有三种模式的特征,以捕获互补信息并实现准确的肿瘤分类。评估使用两个不同的数据集进行:一个用于骨肿瘤检测的临床影像学数据集,以及一个单独的多模式队列,包括用于肿瘤分类的临床影像学、病理学和血液生物标志物。检测模型显示出强大的定位能力,实现了0.7925的测试[email protected]。对于分类任务,消融研究验证了每种模式的互补贡献。值得注意的是,我们的多模态融合方法优于单模态基线,肿瘤分类的宏观平均精度为0.9056,f1评分为0.8736,AUC为0.9759,优于现有模型。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Bone Oncology
ONCOLOGY-
CiteScore
7.20
自引率
2.90%
发文量
50
审稿时长
34 days
期刊介绍:
The Journal of Bone Oncology is a peer-reviewed international journal aimed at presenting basic, translational and clinical high-quality research related to bone and cancer.
As the first journal dedicated to cancer induced bone diseases, JBO welcomes original research articles, review articles, editorials and opinion pieces. Case reports will only be considered in exceptional circumstances and only when accompanied by a comprehensive review of the subject.
The areas covered by the journal include:
Bone metastases (pathophysiology, epidemiology, diagnostics, clinical features, prevention, treatment)
Preclinical models of metastasis
Bone microenvironment in cancer (stem cell, bone cell and cancer interactions)
Bone targeted therapy (pharmacology, therapeutic targets, drug development, clinical trials, side-effects, outcome research, health economics)
Cancer treatment induced bone loss (epidemiology, pathophysiology, prevention and management)
Bone imaging (clinical and animal, skeletal interventional radiology)
Bone biomarkers (clinical and translational applications)
Radiotherapy and radio-isotopes
Skeletal complications
Bone pain (mechanisms and management)
Orthopaedic cancer surgery
Primary bone tumours
Clinical guidelines
Multidisciplinary care
Keywords: bisphosphonate, bone, breast cancer, cancer, CTIBL, denosumab, metastasis, myeloma, osteoblast, osteoclast, osteooncology, osteo-oncology, prostate cancer, skeleton, tumour.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


