Engineering the interfacial water hydrogen-bond network for enhanced alkaline hydrogen evolution
IF 9.7
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The alkaline hydrogen evolution reaction (HER) plays a crucial role in advancing sustainable hydrogen production technologies. However, the limited abundance and reactivity of interfacial water molecules significantly hinder the reaction kinetics. This research develops a WCx-supported PtNi alloy (PtNi/WCx) catalyst to reorganize the hydrogen-bond arrangement of interfacial water by adjusting electron density at metal sites. In situ Raman coupled with ab initio molecular dynamics (AIMD) is employed to investigate how the interfacial hydrogen-bond network influences catalytic activity. These results demonstrate that the electron-rich Pt sites in PtNi/WCx disrupt the hydrogen-bond network by altering the alignment of water molecules. This disruption enhances the mobility and reactivity of H2O* and OH* intermediates at the solid-liquid interface. The optimized PtNi/WCx catalyst achieves exceptional HER activity, requiring only 16 mV at 10 mA cm−2 and 78 mV at 100 mA cm−2. These findings offer a microscopic perspective and theoretical guidelines for optimizing HER catalysts via interfacial water structure manipulation.
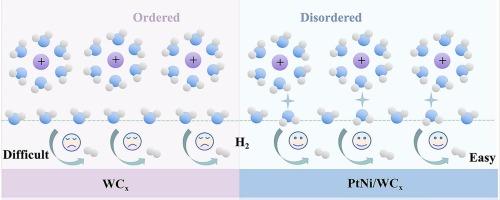
设计界面水氢键网络以增强碱性析氢
碱性析氢反应(HER)在推进可持续制氢技术中起着至关重要的作用。然而,界面水分子的有限丰度和反应活性严重阻碍了反应动力学。本研究开发了一种WCx负载的PtNi合金(PtNi/WCx)催化剂,通过调节金属位的电子密度来重组界面水的氢键排列。采用原位拉曼耦合从头算分子动力学(AIMD)研究了界面氢键网络对催化活性的影响。这些结果表明,PtNi/WCx中的富电子Pt位点通过改变水分子的排列而破坏了氢键网络。这种破坏增强了H2O*和OH*中间体在固液界面的迁移率和反应活性。优化后的PtNi/WCx催化剂具有优异的HER活性,在10 mA cm - 2时仅需16 mV,在100 mA cm - 2时仅需78 mV。这些发现为通过界面水结构调控优化HER催化剂提供了微观视角和理论指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


