1D MonSn Nanowires for Sustainable Hydrogen Evolution: Size and Fermi-Level Driven Catalysis
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
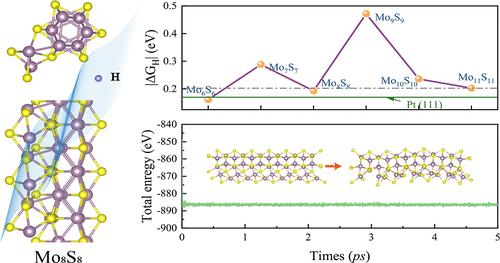
Transition-metal chalcogenide nanowires are promising noble-metal-free catalysts. Their catalytic properties remain underexplored for size and electronic structure tuning. The Mo6S6 nanowire shows well-documented environmental stability. Their structural analogs (MonSn, n = 6–11) lack systematic study. First-principles calculations reveal size-dependent hydrogen evolution reaction (HER) performance in the MonSn nanowires, comparable to platinum. The Mo6S6 nanowire exhibits a hydrogen adsorption free energy of −0.16 eV, similar to Pt(111) at −0.17 eV. The Mo8S8 nanowire, a semiconductor, shows unexpected HER activity despite its larger diameter. Electronic structure analysis indicates hydrogen adsorption triggers a semiconductor-to-metal transition. Strong Mo_4d–H_1s orbital coupling enables dynamic charge redistribution and interfacial conductivity. This metastability overcomes diameter-dependent activity limitations. HER performance in 1D chalcogenides depends on two factors: nanowire diameter affects active-site accessibility, and metal–adsorbate orbital hybridization drives electronic restructuring. The Mo8S8 nanowire combines Pt-competitive activity, ambient stability, and scalable synthesis potential. These findings redefine design principles for low-dimensional HER electrocatalysts. They connect quantum confinement effects with orbital-level reactivity modulation. The results provide a universal strategy for tuning low-dimensional HER catalysts. This approach has implications for transition-metal-based energy conversion systems.
用于可持续析氢的一维蒙锡纳米线:尺寸和费米能级驱动催化
过渡金属硫族纳米线是一种很有前途的无贵金属催化剂。它们的催化性能在尺寸和电子结构调整方面仍未得到充分的研究。Mo6S6纳米线表现出良好的环境稳定性。它们的结构类似物(MonSn, n = 6-11)缺乏系统的研究。第一性原理计算揭示了MonSn纳米线中与尺寸相关的析氢反应(HER)性能,可与铂相媲美。Mo6S6纳米线的氢吸附自由能为- 0.16 eV,与Pt(111)的- 0.17 eV相似。Mo8S8纳米线是一种半导体,尽管它的直径更大,但却显示出意想不到的HER活性。电子结构分析表明,氢吸附触发半导体到金属的转变。强Mo_4d-H_1s轨道耦合可实现动态电荷再分配和界面导电性。这种亚稳态克服了与直径相关的活性限制。一维硫族化合物的she性能取决于两个因素:纳米线直径影响活性位点的可及性,金属-吸附物轨道杂化驱动电子重构。Mo8S8纳米线结合了pt竞争活性、环境稳定性和可扩展的合成潜力。这些发现重新定义了低维HER电催化剂的设计原则。他们将量子约束效应与轨道级反应性调制联系起来。结果为低维HER催化剂的调谐提供了一种通用策略。这种方法对过渡金属基能量转换系统具有启示意义。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


