Binding properties of mononuclear and dinuclear ruthenium(II) complexes toward duplex RNA in both dilute and molecular crowding solutions
IF 2.6
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
Mononuclear ruthenium(II) complex [Ru(bpy)2(tpphz)]2+ (Ru1, bpy = 2,2′-bipyridine, tpphz = tetrapyrido[3,2-a,2′,3′-c,3′′,2′′-h,2′′′,3′′′-j]phenazine) and dinuclear Ru(II) complex [Ru(bpy)2(tpphz)Ru(bpy)2]4+ (Ru2) as RNA binding reagents have been synthesized and characterized in this work, and the binding properties of complexes Ru1 and Ru2 with duplex RNA poly(rA)•poly(rU) in dilute and molecular crowding solutions were studied by spectroscopic methods and viscosity measurements. Luminescence and colorimetric studies show that complexes Ru1 and Ru2 can act as pH-sensitive and reversible naked-eye “molecular light switches” for the studied duplex in both dilute and molecular crowding solutions, while the naked-eye “light switches” response effects and pH-sensitivity of Ru1 are more significant than those of Ru2. The results of spectral titration show that Ru2 has a stronger binding affinity for the duplex and the binding affinities of the two complexes to the duplex are enhanced in molecular crowding solutions. In addition, the possibility that Ru1 and Ru2 interact with the duplex by groove binding and electrostatic interactions is excluded through CD spectra studies, and it is speculated that their binding modes are intercalations (classical intercalation and threading intercalation). The results of equilibrium dialysis experiments indicate that molecular crowding can significantly affect the interaction between the enantiomers of the two Ru(II) complexes and the duplex. Subsequently, the results of thermal denaturation and viscosity measurements provide favorable support for this speculation and show that the complexes Ru1 and Ru2 bind and stabilize the duplex in dilute and molecular crowding solutions through classical intercalation and threading intercalation, respectively, and the ability of the two complexes to bind and stabilize the duplex is enhanced in molecular crowding solutions. To the best of current knowledge, this work represents the first example of stronger binding and stabilizing effects of Ru(II) complexes toward nucleic acids in molecular crowding solutions than in dilute solutions.
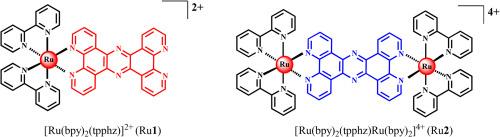
单核和双核钌(II)配合物在稀溶液和分子拥挤溶液中对双工RNA的结合特性
本文合成了单核钌(II)配合物[Ru(bpy)2(tpphz)]2+ (Ru1, bpy = 2,2′-联吡啶,tpphz =四吡啶[3,2-a,2′,3′-c,3′,2′-h,2′,3′-j]苯那嗪]和双核钌(II)配合物[Ru(bpy)2(tpphz)Ru(bpy)2]4+ (Ru2)作为RNA结合试剂,并对其进行了表征,通过光谱方法和粘度测量研究了配合物Ru1和Ru2与双链RNA poly(rA)•poly(Ru)在稀溶液和分子聚集溶液中的结合性能。发光和比色研究表明,络合物Ru1和Ru2在稀溶液和分子拥挤溶液中都可以作为所研究的双相物的ph敏感和可逆的裸眼“分子光开关”,而Ru1的裸眼“光开关”响应效应和ph敏感性比Ru2更显著。光谱滴定结果表明,Ru2对双相具有较强的结合亲和力,在分子拥挤溶液中,两种配合物对双相的结合亲和力增强。此外,通过CD谱研究排除了Ru1和Ru2与双相通过槽结合和静电相互作用的可能性,推测其结合方式为插层(经典插层和螺纹插层)。平衡透析实验结果表明,分子拥挤会显著影响两种Ru(II)配合物对映体与双相的相互作用。随后,热变性和粘度测量的结果为这一推测提供了有利的支持,并表明配合物Ru1和Ru2分别通过经典插层和螺纹插层在稀溶液和分子拥挤溶液中结合和稳定双相,并且这两种配合物在分子拥挤溶液中结合和稳定双相的能力增强。据目前所知,这项工作代表了Ru(II)配合物在分子拥挤溶液中比在稀释溶液中对核酸的结合和稳定作用更强的第一个例子。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Polyhedron
化学-晶体学
CiteScore
4.90
自引率
7.70%
发文量
515
审稿时长
2 months
期刊介绍:
Polyhedron publishes original, fundamental, experimental and theoretical work of the highest quality in all the major areas of inorganic chemistry. This includes synthetic chemistry, coordination chemistry, organometallic chemistry, bioinorganic chemistry, and solid-state and materials chemistry.
Papers should be significant pieces of work, and all new compounds must be appropriately characterized. The inclusion of single-crystal X-ray structural data is strongly encouraged, but papers reporting only the X-ray structure determination of a single compound will usually not be considered. Papers on solid-state or materials chemistry will be expected to have a significant molecular chemistry component (such as the synthesis and characterization of the molecular precursors and/or a systematic study of the use of different precursors or reaction conditions) or demonstrate a cutting-edge application (for example inorganic materials for energy applications). Papers dealing only with stability constants are not considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


