Near-infrared and pH dual-responsive chitosan/epigallocatechin gallate/hollow CuS hydrogel reprograms ROS dynamics for infected wound healing
IF 9.7
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
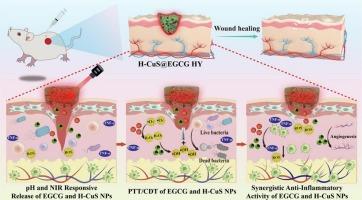
Bacterial-infected wounds pose a clinical challenge requiring innovative strategies integrating precise antibacterial action and microenvironment reprogramming. To address this, a multifunctional H-CuS NPs@epigallocatechin gallate (EGCG)/chitosan (CS)/3-formylphenylboronic acid (3-FPBA) hydrogel (H-CuS@EGCG HY) was engineered. This hydrogel exhibits injectability, self-healing, tissue adhesion, and pH-responsive behavior. During the initial phase of wound infection (weakly acidic microenvironment), this network undergoes controlled dissociation, enabling on-demand localized release of EGCG and Cu1+/2+. Under near-infrared (NIR) irradiation, the photothermal effect of H-CuS NPs promotes mild heating, which enhances EGCG/Cu1+/2+ release and penetration. The released Cu1+/2+ further catalyzes •OH generation from H2O2 via Fenton reaction, synergizing with EGCG and photothermal therapy to improve antibacterial efficacy. Additionally, EGCG scavenges excess radicals, reducing oxidative stress and inflammation, while Cu1+/2+ supports angiogenesis via VEGF regulation-collectively reprogramming the wound microenvironment. In vitro tests showed broad-spectrum antimicrobial activity with a 99 % eradication rate. In S. aureus-infected mouse models, compared to the control group, the H-CuS@EGCG HY + NIR group significantly reduced the levels of inflammatory cytokines (TNF-α), promoted the expression of CD31 and CD206, and effectively modulated the wound microenvironment, ultimately resulting in complete wound closure (100 %) by day 13, accompanied by full epidermal regeneration and minimal scarring. This study provides a promising strategy for the treatment of clinical wounds.
近红外和pH双响应壳聚糖/表没食子儿茶素没食子酸酯/空心CuS水凝胶重编程ROS动力学用于感染伤口愈合
细菌感染的伤口是一项临床挑战,需要整合精确抗菌作用和微环境重编程的创新策略。为此,设计了一种多功能h - cu NPs@epigallocatechin没食子酸酯(EGCG)/壳聚糖(CS)/3-甲酰基苯硼酸(3-FPBA)水凝胶(H-CuS@EGCG HY)。这种水凝胶具有可注射性、自愈性、组织粘连性和ph响应性。在伤口感染的初始阶段(弱酸性微环境),该网络经历受控解离,使EGCG和Cu1+/2+按需局部释放。在近红外(NIR)照射下,h - cu NPs光热效应促进了轻度加热,促进了EGCG/Cu1+/2+的释放和渗透。释放的Cu1+/2+进一步通过Fenton反应催化H2O2生成•OH,与EGCG和光热疗法协同作用,提高抗菌效果。此外,EGCG清除多余的自由基,减少氧化应激和炎症,而Cu1+/2+通过VEGF调节支持血管生成,共同重新编程伤口微环境。体外试验显示广谱抗菌活性,根除率达99%。在金黄色葡萄球菌感染的小鼠模型中,与对照组相比,H-CuS@EGCG HY + NIR组显著降低了炎症因子(TNF-α)水平,促进了CD31和CD206的表达,并有效调节了创面微环境,最终导致创面在第13天完全闭合(100%),并伴有表皮完全再生和最小疤痕。本研究为临床创伤的治疗提供了一个有希望的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
16.10
自引率
7.10%
发文量
2568
审稿时长
2 months
期刊介绍:
The Journal of Colloid and Interface Science publishes original research findings on the fundamental principles of colloid and interface science, as well as innovative applications in various fields. The criteria for publication include impact, quality, novelty, and originality.
Emphasis:
The journal emphasizes fundamental scientific innovation within the following categories:
A.Colloidal Materials and Nanomaterials
B.Soft Colloidal and Self-Assembly Systems
C.Adsorption, Catalysis, and Electrochemistry
D.Interfacial Processes, Capillarity, and Wetting
E.Biomaterials and Nanomedicine
F.Energy Conversion and Storage, and Environmental Technologies

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


