Synthesis of C3 and C5 Oxygenates from the Co-electrolysis of Carbon Monoxide and Acetylene on a Cu–Pd Catalyst
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The electroreduction of CO2 (CO2RR) and CO (CORR) using renewable electricity provides a sustainable route for decarbonizing chemical manufacturing. However, boosting the production of multi-carbon oxygenates remains challenging. Herein, we report that the co-electroreduction of CO and acetylene (C2H2) on an oxide-derived Cu5Pd (OD-Cu5Pd) catalyst at −1.1 V vs SHE achieves ∼130× higher production of C3 oxygenates, such as 1-propanol, propionaldehyde, and allyl alcohol, compared to CORR alone. Seven C5 oxygenates, including 3-pentanone, cyclopentanone, and 1-pentanol, were further identified, none of which have been previously observed in either CORR or CO2RR. The remaining balance of the products was mainly ethylene and 1,3-butadiene. The addition of Pd to Cu enhances both CO and C2H2 adsorption, facilitates C–C coupling, and significantly improves the oxygenate yields. At −1.1 V vs SHE, the production rates of C3 oxygenates on OD-Cu5Pd catalyst were doubled, while that of C5 oxygenates increased by ∼6-fold, as compared to that on unmodified OD-Cu. The oxygenate production rates were further optimized by tuning the applied potential, feedstock stoichiometry, and temperature. Mechanistic studies indicate that the C3 oxygenates and the C5 oxygenates with mid-chain oxygen atoms form via CO and C2H2 coupling, while the C5 oxygenates with terminal oxygen atoms stem from CO reacting with 1,3-butadiene, a C2H2 reduction intermediate. These results present a promising and sustainable strategy for the electrosynthesis of long-chain oxygenates.
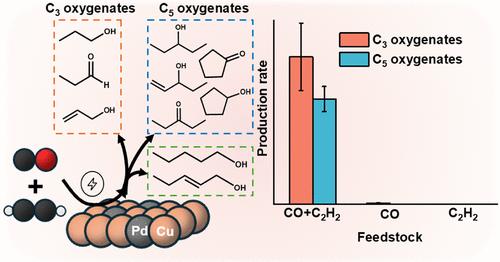
在Cu-Pd催化剂上一氧化碳和乙炔共电解合成C3和C5氧合物
利用可再生电力电还原CO2 (CO2RR)和CO (CORR)为化工生产脱碳提供了一条可持续的途径。然而,促进多碳氧化合物的生产仍然具有挑战性。在此,我们报告了CO和乙炔(C2H2)在氧化物衍生的Cu5Pd (OD-Cu5Pd)催化剂上在−1.1 V vs SHE下的共电还原,与单独CORR相比,C3氧合物(如1-丙醇、丙醛和烯丙醇)的产量提高了约130倍。进一步鉴定了7种C5含氧化合物,包括3-戊酮、环戊酮和1-戊醇,这些化合物在CORR或CO2RR中均未被发现。剩余产物主要为乙烯和1,3-丁二烯。在Cu中添加Pd可增强CO和C2H2的吸附,促进C-C偶联,显著提高含氧产物收率。在−1.1 V vs SHE条件下,OD-Cu5Pd催化剂上C3氧合物的生成速率比未修饰的OD-Cu催化剂提高了一倍,而C5氧合物的生成速率提高了约6倍。通过调节应用电位、原料化学计量和温度,进一步优化了氧化产物的产率。机理研究表明,C3型氧合物和中链氧原子的C5型氧合物是CO与C2H2偶联形成的,而末端氧原子的C5型氧合物是CO与C2H2还原中间体1,3-丁二烯反应形成的。这些结果为电合成长链含氧化合物提供了一个有前途和可持续的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


