Reversible Enzymatic Switching of the Oxidation State of a EuIII/II Complex Controls Relaxivity
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
We show that europium trisbipyridyl cryptate exhibits an electrochemically reversible EuIII/II redox couple at −360 mV vs the standard hydrogen electrode (SHE) and is susceptible to reduction or oxidation by a range of redox enzymes or biological small molecule redox agents. Modulation of the europium oxidation state can be used to achieve dramatic changes in the T1 relaxation time of bulk water in solutions containing these complexes: in the reduced form, the complex exhibits high relaxivity (3.08 mM–1 s–1 at 499.9 MHz) comparable with that of commercial MRI contrast media, while the oxidized form has negligible relaxivity, illustrating the scope for application in imaging reducing environments in biological systems.
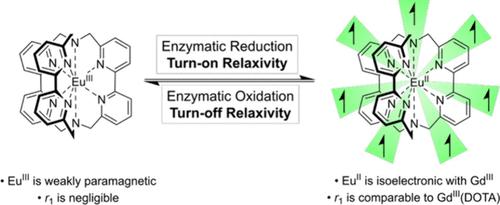
EuIII/II络合物氧化态的可逆酶开关控制弛豫
我们发现,与标准氢电极(SHE)相比,三联吡啶酸铕在−360 mV下表现出电化学可逆的EuIII/II氧化还原偶,并且容易被一系列氧化还原酶或生物小分子氧化还原剂还原或氧化。铕氧化态的调制可以用来实现含有这些配合物的溶液中大块水的T1弛豫时间的巨大变化:在还原形式下,配合物表现出与商用MRI造影剂相当的高弛豫(3.08 mM-1 s-1, 499.9 MHz),而氧化形式的弛豫可以忽略不计,说明了在生物系统成像还原环境中的应用范围。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


