Biocatalytic Stereodivergent Construction of Axially Chiral Tri- and Tetrasubstituted Allenols via Desymmetric Hydroxylation
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Axially chiral allenes serve as versatile building blocks in organic synthesis and are important motifs in natural products. While enantioselective syntheses of disubstituted allenes have been well-established, catalytic construction of trisubstituted and tetrasubstituted allenes with high enantiopurity remains a formidable challenge. In this study, we present a biocatalytic strategy for the stereodivergent synthesis of axially chiral tri- and tetrasubstituted allenols via engineered P450pyr monooxygenase-catalyzed desymmetric hydroxylation of C(sp3)–H bonds. Through structure-guided semirational mutagenesis, enantiocomplementary P450pyr variants were developed to deliver both (R)- and (S)-configured allenols in a highly regio-, chemo-, and enantioselective manner (up to 99% ee). Molecular docking studies elucidated the structural basis for the enhanced and inverted stereocontrol in the engineered mutants, correlating active-site interactions with stereochemical outcomes. This enzymatic platform represents the first example of P450-catalyzed hydroxylation for constructing axially chiral tri- and tetrasubstituted allenes that are otherwise challenging to access.
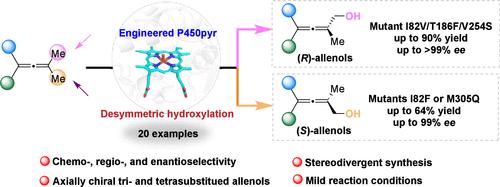
轴手性三取代和四取代烯醇的非对称羟基化生物催化立体发散结构
轴向手性烯在有机合成中起着多用途的基础作用,是天然产物的重要基序。虽然双取代烯的对映选择性合成已经建立,但具有高对映纯度的三取代和四取代烯的催化构建仍然是一个巨大的挑战。在这项研究中,我们提出了一种生物催化策略,通过工程P450pyr单加氧酶催化C(sp3) - h键的不对称羟基化,实现轴手性三取代和四取代烯醇的立体发散合成。通过结构引导的半分子诱变,P450pyr对映体互补变体被开发出来,以高度区域选择性、化学选择性和对映选择性的方式传递(R)和(S)配置的等位醇(高达99% ee)。分子对接研究阐明了工程突变体增强和倒置立体控制的结构基础,将活性位点相互作用与立体化学结果联系起来。该酶平台代表了p450催化羟基化的第一个例子,用于构建轴向手性三取代和四取代烯,否则难以获得。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


