Manipulating Interfacial Charge Distribution for Water Reduction
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Heterogeneous electrocatalysis often involves a complex interplay of the non-Faradaic process and the Faradaic process at the electrode/electrolyte interface, with the reaction kinetics typically exhibiting an exponential dependence on applied bias. However, the indivisible nature of bias hinders independent understanding and fine-tuning of each process. Synergistically regulating these two processes containing multiple reaction steps via one single, yet unified mediator remains a formidable challenge. Here, precise manipulation of the interfacial charge distribution is proposed to tackle this critical challenge. Through lattice strain engineering in NiCo2S4, the optimal amount matching between non-Faradaic charges and Faradaic charges is yielded during alkaline hydrogen evolution reaction (HER). As a result, the restriction of electrostatic potential on the water molecule reorganization process is alleviated, and the positive contribution of chemical potential to the water molecule dissociation process is concurrently strengthened. Furthermore, the moderate eg orbital filling is evidenced as the essential origin of a substantial increase in Faraday charges, the relationship between which is in the volcanic form. This interfacial charge redistribution enabled a synergistic optimization route of kinetic multisteps that can be further extended to other heterogeneous electrocatalytic reactions beyond HER.
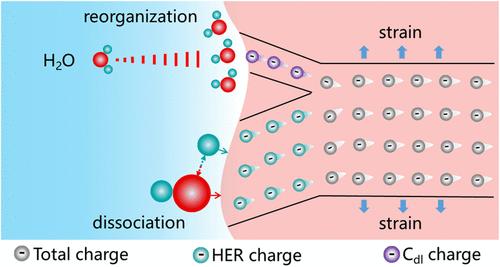
控制界面电荷分布以减少水
非均相电催化通常涉及电极/电解质界面上非法拉第过程和法拉第过程的复杂相互作用,反应动力学通常表现出对施加偏倚的指数依赖。然而,偏见的不可分割性阻碍了对每个过程的独立理解和微调。协同调节这两个过程包含多个反应步骤通过一个单一的,但统一的中介仍然是一个艰巨的挑战。在这里,提出了对界面电荷分布的精确操纵来解决这一关键挑战。通过NiCo2S4的晶格应变工程,在碱性析氢反应(HER)中获得了非法拉第电荷与法拉第电荷的最佳匹配量。从而减轻了静电势对水分子重组过程的限制,同时加强了化学势对水分子解离过程的积极贡献。此外,适度的eg轨道填充被证明是法拉第电荷大量增加的根本原因,两者之间的关系是火山形式的。这种界面电荷重分配使得动力学多步骤的协同优化路线可以进一步扩展到she以外的其他非均相电催化反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


