TET2-mutant clonal hematopoiesis enhances macrophage antigen presentation and improves immune checkpoint therapy in solid tumors
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
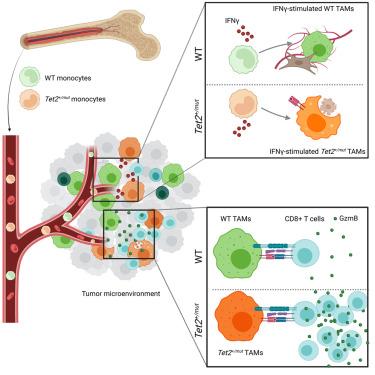
Clonal hematopoiesis (CH) is detectable in upwards of 20% of patients with solid tumors and is associated with worsened prognosis; however, its role in tumor immunology and immune checkpoint therapy (ICT) is unknown. Using a bone marrow chimera model of Tet2+/mut CH in mice with solid tumors, we found the Tet2-mutant myeloid cells are abundant in the tumor microenvironment and contributed to an improved response to ICT. Mechanistically, Tet2+/mut macrophages inside the tumor act as immunogenic antigen-presenting cells that more effectively cross-prime naive CD8+ T cells in response to IFNγ. In human cohorts of 35,971 non-small cell lung cancer patients and 25,064 colorectal adenocarcinoma patients, TET2-mutant CH is associated with improved outcome specifically with ICT. This study proposes a role for Tet2+/mut antigen presenting macrophages in shaping antitumor immunity and identifies TET2-mutant CH as a potential biomarker for improved response to ICT in patients with solid tumors.
tet2突变克隆造血增强巨噬细胞抗原呈递和改善实体瘤免疫检查点治疗
克隆性造血(CH)在20%以上的实体瘤患者中可检测到,并与预后恶化有关;然而,其在肿瘤免疫学和免疫检查点治疗(ICT)中的作用尚不清楚。利用实体瘤小鼠的Tet2+/mut CH骨髓嵌合体模型,我们发现Tet2突变的骨髓细胞在肿瘤微环境中大量存在,并有助于改善对ICT的反应。在机制上,肿瘤内的Tet2+/mut巨噬细胞作为免疫原性抗原呈递细胞,更有效地交叉初始CD8+ T细胞以响应IFNγ。在35,971例非小细胞肺癌患者和25,064例结直肠腺癌患者的人类队列中,tet2突变CH与ICT改善的预后相关。本研究提出Tet2+/mut抗原呈递巨噬细胞在形成抗肿瘤免疫中的作用,并确定Tet2突变CH作为实体瘤患者对ICT反应改善的潜在生物标志物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


