Li1.75Zr0.75Sb0.25Cl4.75O0.625 Oxyhalide Solid Electrolyte: Enhancing Performance by Regulating Phase Transition
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
New types of halides have garnered significant attention from researchers in the development of high-performance all-solid-state lithium-ion batteries due to their exceptional high-potential stability, high ionic conductivity, and favorable mechanical properties. Here, a mechanochemical ball milling method is employed to synthesize the Li2–2xZr1–2xSb2xCl6–10xO5x material by incorporating an appropriate amount of Sb–O into Li2ZrCl6. Experimental results and Rietveld refinement confirm the successful synthesis of a zirconium-based chloride-oxide solid electrolyte material (Li1.75Zr0.75Sb0.25Cl4.75O0.625). Due to the incorporation of Sb5+, which has a smaller ionic radius but a higher valence state, the volume of the condensed crystal lattice decreased, while the number of lithium vacancies increased. The incorporation of O2– induced a phase transition in the crystal structure, leading to the redistribution of lithium ions. The combined effects of these factors enhanced the ionic conductivity. The lithium-ion conductivity of the electrolyte at 25 °C is 4.2 × 10–4 S cm–1, and it possesses a wide electrochemical stability window (1.22–4.62 V vs Li+/Li). Simultaneously, Sb–O dual doping enhances the electrochemical reduction stability of Li2ZrCl6. The all-solid-state battery utilizing Li1.75Zr0.75Sb0.25Cl4.75O0.625 as the electrolyte and sc-NCM811 as the cathode demonstrates excellent cycling performance and a high capacity retention rate. These investigations offer a promising strategy for the rational design of zirconium-based halide solid-state electrolytes, which are essential for the development of high-performance all-solid-state batteries.
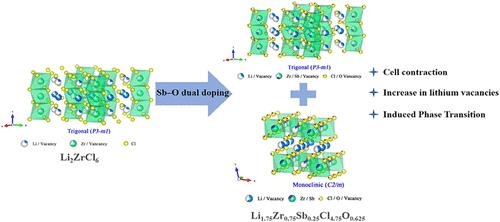
Li1.75Zr0.75Sb0.25Cl4.75O0.625氧化卤化物固体电解质:通过调节相变提高性能
新型卤化物由于其优异的高电位稳定性、高离子电导率和良好的机械性能,在高性能全固态锂离子电池的开发中引起了研究人员的极大关注。在Li2ZrCl6中加入适量的Sb-O,采用机械化学球磨法制备Li2-2xZr1-2xSb2xCl6-10xO5x材料。实验结果和Rietveld细化证实了锆基氯氧化物固体电解质材料(Li1.75Zr0.75Sb0.25Cl4.75O0.625)的成功合成。由于离子半径较小但价态较高的Sb5+的掺入,使得缩合晶格的体积减小,而锂空位的数量增加。O2 -的加入引起了晶体结构的相变,导致锂离子的重新分布。这些因素的共同作用增强了离子电导率。电解质在25°C时的锂离子电导率为4.2 × 10-4 S cm-1,具有较宽的电化学稳定窗口(1.22 ~ 4.62 V vs Li+/Li)。同时,Sb-O双掺杂增强了Li2ZrCl6的电化学还原稳定性。以Li1.75Zr0.75Sb0.25Cl4.75O0.625为电解液,sc-NCM811为阴极的全固态电池具有良好的循环性能和较高的容量保持率。这些研究为合理设计锆基卤化物固态电解质提供了一种有希望的策略,这对于高性能全固态电池的发展至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


