Decoding Diluent-Driven Solvation Dynamics in Locally Concentrated Ionic Liquid Electrolytes
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
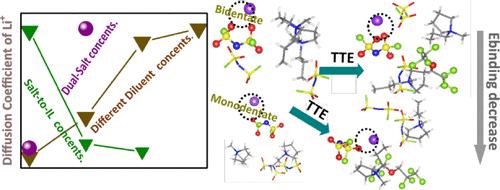
The interplay between lithium salts and anions critically influences the electrochemical and physicochemical properties of locally concentrated ionic liquid electrolytes (LCILEs), a promising class of materials for next-generation lithium-ion batteries. Here, we investigate how varying diluent concentrations modulate lithium–anion interactions, ion dynamics, and transport properties in LCILEs. Using molecular dynamics (MD) simulations combined with density functional theory (DFT) calculations, we show that incorporating 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropyl ether (TTE) as a diluent minimally perturbs the solvation structure while effectively weakening Li+–anion interactions and promoting ionic dissociation. Adjusting the lithium salt-to-ionic liquid (IL) ratio alters the coordination environment of bis(fluorosulfonyl)imide anions (FSI–). It reduces the presence of pyrrolidinium cations in the lithium solvation shell. Beyond solvation effects, we further demonstrate that introducing lithium hexafluorophosphate (LiPF6) enhances ionic conductivity and increases the lithium-ion diffusion coefficient. By systematically exploring the impacts of diluent concentration and ionic additives, our theoretical framework offers molecular-level insights into how electrolyte composition influences lithium-ion mobility and interfacial stability, key factors in designing high-performance electrolytes for next-generation energy storage systems.
解析局部浓缩离子液体电解质中稀释剂驱动的溶剂化动力学
锂盐和阴离子之间的相互作用严重影响局部浓缩离子液体电解质(liles)的电化学和物理化学性质,liles是下一代锂离子电池的一种有前途的材料。在这里,我们研究了不同稀释剂浓度如何调节liles中的锂阴离子相互作用、离子动力学和输运性质。通过分子动力学(MD)模拟和密度泛函理论(DFT)计算,我们发现1,1,2,2,3,3 -四氟乙基-2,2,3,3-四氟丙基醚(TTE)作为稀释剂对溶剂化结构的干扰最小,同时有效地减弱Li+ -阴离子相互作用并促进离子解离。调整锂盐与离子液体(IL)的比例可以改变双(氟磺酰基)亚胺阴离子(FSI -)的配位环境。它减少了锂溶剂化壳层中吡啶离子的存在。除了溶剂化效应,我们进一步证明了引入六氟磷酸锂(LiPF6)可以提高离子电导率,增加锂离子扩散系数。通过系统地探索稀释剂浓度和离子添加剂的影响,我们的理论框架为电解质成分如何影响锂离子迁移率和界面稳定性提供了分子水平的见解,这是设计下一代储能系统高性能电解质的关键因素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


