Hijacking Extracellular Targeted Protein Degrader–Drug Conjugates for Enhanced Drug Delivery
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Antibody-based therapeutics encompass diverse modalities for targeting tumor cells. Among these, antibody–drug conjugates (ADCs) and extracellular targeted protein degradation (eTPD) specifically depend on efficient lysosomal trafficking for activity. A major limitation of ADCs is their reliance on antigens with efficient internalization, while eTPD approaches, although capable of trafficking diverse targets to lysosomes, lack cytotoxic potency. To address this, we developed degrader–drug conjugates (DDCs), leveraging the endocytic and recycling activities of eTPD to enhance lysosomal delivery. We utilized fast internalizers, the low-density lipoprotein receptor (LDLR) and the chemokine receptor (CXCR7), to enhance lysosomal delivery. LDLR-based degraders enabled efficient and selective degradation of diverse extracellular membrane proteins, while DDCs with cytotoxic payload enhanced cytotoxicity compared to conventional ADCs in vitro. This dual modality addresses key challenges of inadequate internalization in conventional ADCs and cytotoxic potency in current eTPD strategies. Our findings demonstrate that DDCs provide additional optionality for developing next-generation antibody therapeutics with broader utility and improved efficacy in cancer treatment.
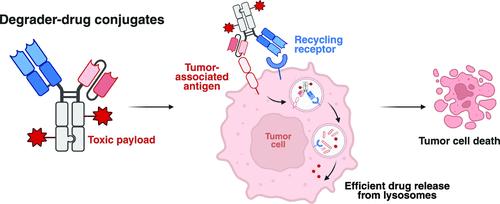
劫持细胞外靶向蛋白质降解-药物偶联物以增强药物传递
基于抗体的治疗方法包括针对肿瘤细胞的多种方式。其中,抗体-药物偶联物(adc)和细胞外靶向蛋白降解(eTPD)特别依赖于高效的溶酶体运输来获得活性。adc的一个主要限制是它们依赖于有效内化的抗原,而eTPD方法虽然能够将多种靶标运输到溶酶体,但缺乏细胞毒性。为了解决这个问题,我们开发了降解药物偶联物(ddc),利用eTPD的内吞和再循环活性来增强溶酶体的递送。我们利用快速内化剂,低密度脂蛋白受体(LDLR)和趋化因子受体(CXCR7)来增强溶酶体的递送。基于ldlr的降解剂能够有效和选择性地降解多种胞外膜蛋白,而与传统adc相比,具有细胞毒性载荷的ddc在体外增强了细胞毒性。这种双重模式解决了传统adc内化不足和当前eTPD策略中细胞毒性效力不足的关键挑战。我们的研究结果表明,ddc为开发下一代抗体疗法提供了额外的选择,在癌症治疗中具有更广泛的用途和更高的疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


