Ni-Catalyzed Enantioselective Arylboration of 1,3-Cyclohexadiene
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The development of straightforward and efficient methodologies for synthesizing compounds featuring chiral six-membered ring scaffolds represents a crucial pursuit within the realm of synthetic chemistry. Herein, we report a Ni-catalyzed regio-, enantio-, and diastereoselective arylboration of 1,3-cyclohexadienes employing readily available bis(pinacolato)diboron (B2pin2) and aryl iodides, providing convenient access to synthetically valuable chiral boronated 1,4-cis-cyclohexenes with high levels of regio-, enantio-, and diastereoselectivity. Notably, the mild reaction conditions ensure broad functional group compatibility. This has been demonstrated by the broad substrate scope and late-stage functionalizations of bioactive compounds or drug molecules within the reaction system. Moreover, the functionally diverse chiral boronated 1,4-cis-cyclohexene derivatives demonstrate high reactivity in a range of downstream transformations, allowing for the efficient preparation of various enantio-enriched, multisubstituted six-membered ring compounds, thereby underscoring the importance of preserving both the boron moiety and the double bond. Preliminary mechanistic investigations, supported by density functional theory (DFT) calculations, provide insights into the origins of the observed regio- and enantioselectivity.
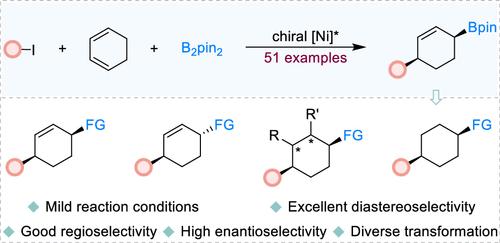
镍催化1,3-环己二烯芳基化反应
开发直接有效的方法来合成具有手性六元环支架的化合物是合成化学领域的一个重要追求。在这里,我们报道了镍催化的1,3-环己二烯的区域、对映体和非对映体选择性芳基硼化反应,利用现成的双(pinacolato)二硼(B2pin2)和芳基碘化物,提供了方便的合成有价值的手性硼化1,4-顺式环己烯,具有高水平的区域、对映体和非对映体选择性。值得注意的是,温和的反应条件确保了广泛的官能团相容性。这已经被反应系统中广泛的底物范围和生物活性化合物或药物分子的后期功能化所证明。此外,功能多样的手性硼化1,4-顺式环己烯衍生物在一系列下游转化中表现出较高的反应活性,从而可以有效地制备各种富含对映体的多取代六元环化合物,从而强调了保留硼部分和双键的重要性。在密度泛函理论(DFT)计算的支持下,初步的机制研究为观察到的区域和对映体选择性的起源提供了见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


