A Sodium Iron Phosphate Nanocatalyst-Regulated High-Mobility Ion Reservoir Enables Tumor-Selective Immunotherapy
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Sodium ion (Na+)-mediated pyroptosis has emerged as a promising strategy in ion interference therapy (IIT) for tumor treatment. However, conventional Na+ delivery systems suffer from off-target leakage and osmotic toxicity in normal tissues. Herein, we present the construction of a peroxidase-mimicking ion reservoir based on sodium iron phosphate (NFPP) nanocatalysts, which enables rapid and tumor-selective Na+ outflow through pH-gated nanocatalytic activation. NFPP maintains an “OFF” state in normal cells due to the minimal ion release induced by the catalytic inactivation. Crucially, in tumor cells, the peroxidase-like catalytic activity of NFPP is activated by the more acidic lysosome environment than that within normal cells, transitioning NFPP to an “ON” state. The optimal activity boosts the Na+ outward migration, resulting in intracellular Na+ overload that triggers the pyroptosis pathway. Conversely, the rapid Na+ flux amplifies the peroxidase-like catalytic activity, thus reinforcing oxidative stress and exacerbating pyroptosis. The NFPP nanoplatform can further provoke robust immune responses and provide long-term protection against tumor relapse and metastasis. This work pioneers a design strategy for constructing nanocatalysis-regulated high-mobility ion reservoirs to overcome limitations in precise ion modulation for selective tumor immunotherapy.
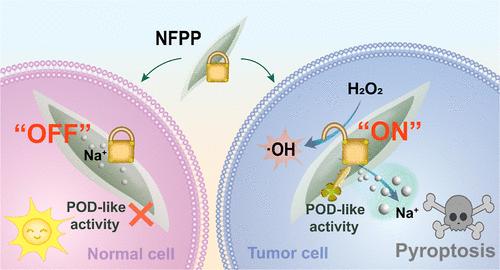
磷酸铁钠纳米催化剂调控高迁移率离子库实现肿瘤选择性免疫治疗
钠离子(Na+)介导的焦亡已成为离子干扰治疗(IIT)治疗肿瘤的一种很有前途的策略。然而,传统的Na+传递系统在正常组织中存在脱靶泄漏和渗透毒性。在这里,我们提出了一个基于磷酸铁钠(NFPP)纳米催化剂的过氧化物酶模拟离子库的构建,它可以通过ph门控纳米催化激活快速和肿瘤选择性地流出Na+。NFPP在正常细胞中保持“关闭”状态,这是由于催化失活诱导的最小离子释放。至关重要的是,在肿瘤细胞中,NFPP的过氧化物酶样催化活性被比正常细胞中酸性更强的溶酶体环境激活,将NFPP转变为“ON”状态。最佳活性促进Na+向外迁移,导致细胞内Na+过载触发焦亡途径。相反,快速的Na+通量放大了过氧化物酶样的催化活性,从而增强氧化应激,加剧焦亡。NFPP纳米平台可以进一步激发强大的免疫反应,并提供长期保护,防止肿瘤复发和转移。这项工作开创了构建纳米催化调节的高迁移率离子储存器的设计策略,以克服选择性肿瘤免疫治疗中精确离子调节的局限性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


