Chemical Engineering of Transcription Factors Uncovered Cell-Permeable μMax Modulators
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
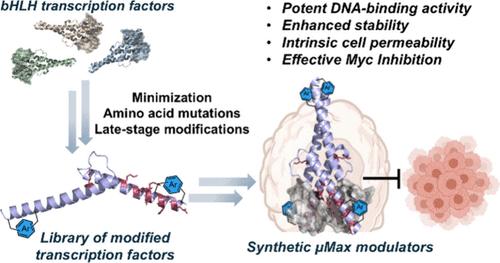
Transcription factor engineering has emerged as a powerful strategy for generating novel proteins for fundamental research and biomedical applications. Although various analogs have been developed, they remain largely constrained to native sequences and structures. The generation of advanced analogs bearing noncanonical modifications with enhanced functional properties remains limited. Here we combined rational design with total synthesis to engineer novel abiotic transcription factors with enhanced stability and cell permeability. Using solid-phase synthesis and native chemical ligation, we created a library of 30 Max-derived transcription factor analogs incorporating novel modifications, such as sequence mutations and aromatic staples at strategic sites. Through DNA-binding analysis and cellular uptake studies, we identified the μMax20 analog, which contains two mutations (Lys31 and Lys57 to hArg) and exhibits potent DNA binding to the canonical enhancer box (E-box) as well as intrinsic cell permeability. Notably, further site-specific modifications of μMax20 with aromatic staples yielded improved analogs with enhanced stability and remarkable cellular delivery at nanomolar concentrations. Our lead μMax20 analog suppressed Myc-driven gene expression, as demonstrated by reporter gene assays and antiproliferative activity against Myc-dependent cancer cells. Altogether, these results highlight how combining chemical protein synthesis with late-stage modifications can be leveraged to enhance protein function and engineer novel bioactive modulators.
转录因子的化学工程揭示细胞渗透性μMax调节剂
转录因子工程已经成为基础研究和生物医学应用中产生新型蛋白质的有力策略。虽然已经开发了各种类似物,但它们在很大程度上仍然局限于原生序列和结构。具有增强功能特性的非规范修饰的高级类似物的产生仍然有限。在这里,我们将合理设计与全合成相结合,设计出具有增强稳定性和细胞渗透性的新型非生物转录因子。利用固相合成和天然化学连接,我们创建了一个包含30个max衍生的转录因子类似物的文库,这些转录因子类似物包含了新的修饰,如序列突变和战略性位点的芳香钉。通过DNA结合分析和细胞摄取研究,我们鉴定出μMax20类似物,该类似物包含两个突变(Lys31和Lys57对hArg),并表现出与典型增强子盒(E-box)的有效DNA结合以及固有的细胞渗透性。值得注意的是,对μMax20进行进一步的位点特异性修饰,得到的类似物具有更强的稳定性和在纳摩尔浓度下显著的细胞递送。我们的先导μMax20类似物抑制myc驱动基因的表达,通过报告基因检测和对myc依赖性癌细胞的抗增殖活性证实了这一点。总之,这些结果强调了如何结合化学蛋白质合成和后期修饰来增强蛋白质功能和设计新的生物活性调节剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


