Bioinspired Carbonic Anhydrase Mimics with Zr–OH Sites and Size-Tunable Nanopockets for Efficient Bidirectional Catalysis of CO2 Hydration
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Carbonic anhydrase (CA)-mimetic enzymes display promising potential in CO2-related catalytic applications, whereas currently designed CA mimics are limited to mimicking the active site and nanopockets. Here, we report the de novo design of bioinspired Zr–OH sites and size-tunable nanopockets via Zr-organic framework structures for the efficient catalysis of CO2 hydration and HCO3– dehydration. Competitive coordination modulation enhances Zr–OH site density and accessibility. The high connectivity of the ligand increases the sensitivity of the Zr-organic framework to ligand defects, while its inherently large size provides near-mesoporous-scale nanopockets. This design enables exceptional 4-nitrophenyl acetate hydrolysis performance (Vmax = 3.86 μM s–1 and TON = 29.92 × 10–3 s–1), superior to the state-of-the-art CA mimics. Meanwhile, we systematically elucidate the catalytic mechanism of Zr–OH and show that it interacts with CO2 to form bidentate carbonate intermediates─similar to the catalytic mechanism of natural CA. Furthermore, the Zr-organic framework can also catalyze the dehydration of bicarbonate, which is consistent with the bidirectional character of natural CA. This work provides fundamental insights into the design of atomic sites and the regulation of nanopockets in enzyme mimics, as well as a promising strategy for preparing high-performance CA mimics for CO2-related applications.
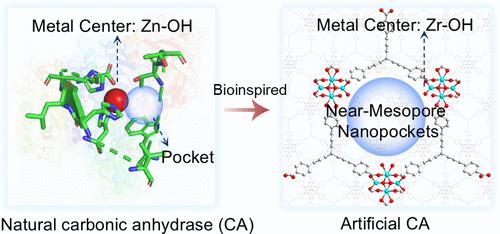
仿生碳酸酐酶模拟与Zr-OH位点和大小可调的纳米孔有效双向催化二氧化碳水化
碳酸酐酶(CA)模拟酶在二氧化碳相关的催化应用中显示出很大的潜力,而目前设计的CA模拟酶仅限于模拟活性位点和纳米孔。在这里,我们报道了通过zr -有机框架结构重新设计的仿生Zr-OH位点和尺寸可调的纳米孔,用于有效催化CO2水化和HCO3 -脱水。竞争配位调制提高了Zr-OH位点密度和可及性。配体的高连通性增加了zr -有机骨架对配体缺陷的敏感性,而其固有的大尺寸提供了近介孔尺度的纳米孔。该设计具有优异的4-硝基苯醋酸酯水解性能(Vmax = 3.86 μM s-1, TON = 29.92 × 10-3 s-1),优于最先进的CA模拟物。同时,我们系统地阐明了Zr-OH的催化机理,发现Zr-OH与CO2相互作用形成双齿碳酸盐中间体,与天然CA的催化机理相似。此外,zr -有机骨架还可以催化碳酸氢盐脱水,这与天然CA的双向特性一致。这项工作为模拟酶中原子位点的设计和纳米孔的调控提供了基础见解。以及为二氧化碳相关应用程序准备高性能CA模拟的有前途的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


