NSD2 exacerbates metabolic dysfunction-associated steatotic liver disease progression by suppressing TFEB-mediated autophagy-lysosomal pathway
IF 11.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Objectives
Impaired autophagy is increasingly recognized as a key contributor to the pathogenesis of metabolic dysfunction-associated steatotic liver disease (MASLD). However, its underlying molecular mechanisms remain largely undefined. Emerging evidence implicates epigenetic regulators in modulating autophagic pathways in metabolic diseases. Therefore, this study aimed to elucidate the role of a histone methyltransferase, nuclear receptor binding SET domain protein 2 (NSD2), in regulating autophagy and its contribution to MASLD progression.
Methods
NSD2 expression levels were evaluated in liver tissues from patients with MASLD and mouse models. Functional studies were conducted using hepatocyte-specific Nsd2 knockout and overexpression mouse models, along with cleavage under targets and tagmentation analysis in hepatocyte cell lines. Additionally, the effects of pharmacological NSD2 inhibition using NSC663284 were evaluated in human liver organoids. Autophagy, hepatic steatosis, and related epigenetic changes were assessed through molecular and histological techniques.
Results
NSD2 expression was markedly elevated in both patient livers and murine models, correlating positively with disease severity. Hepatic NSD2 deficiency alleviated diet-induced autophagy impairment and steatosis, while NSD2 overexpression exacerbated these pathologies. Mechanistically, NSD2 epigenetically suppressed TFEB transcription by promoting trimethylation of histone H4 at lysine 20, impairing autophagy. Pharmacological inhibition of NSD2 with NSC663284 similarly alleviated hepatic steatosis in human liver organoids.
Conclusion
NSD2 acts as a key epigenetic suppressor of TFEB-mediated autophagy in the liver, promoting lipid accumulation and MASLD progression. Targeting NSD2 represents a promising therapeutic strategy for MASLD.
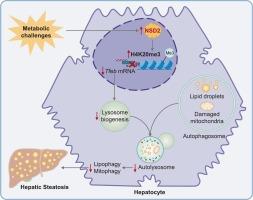
NSD2通过抑制tfeb介导的自噬-溶酶体途径加剧代谢功能障碍相关的脂肪变性肝病进展。
目的:受损的自噬越来越被认为是代谢功能障碍相关脂肪变性肝病(MASLD)发病机制的关键因素。然而,其潜在的分子机制在很大程度上仍未明确。新出现的证据暗示表观遗传调节剂在代谢疾病中调节自噬途径。因此,本研究旨在阐明组蛋白甲基转移酶核受体结合SET结构域蛋白2 (NSD2)在调节自噬及其对MASLD进展的贡献中的作用。方法:检测MASLD患者肝组织及小鼠模型中NSD2的表达水平。使用肝细胞特异性Nsd2敲除和过表达小鼠模型进行功能研究,并在肝细胞细胞系中进行靶向切割和标记分析。此外,我们还评估了NSC663284对人肝类器官NSD2的药理抑制作用。通过分子和组织学技术评估自噬、肝脂肪变性和相关的表观遗传改变。结果:NSD2在患者肝脏和小鼠模型中的表达均显著升高,且与疾病严重程度呈正相关。肝脏NSD2缺乏可减轻饮食诱导的自噬损伤和脂肪变性,而NSD2过表达则加重了这些病理。机制上,NSD2通过促进赖氨酸20位点组蛋白H4的三甲基化来抑制TFEB转录,从而损害自噬。NSC663284对NSD2的药理抑制同样减轻了人肝类器官的肝脂肪变性。结论:NSD2是tfeb介导的肝脏自噬的关键表观遗传抑制因子,促进脂质积累和MASLD进展。靶向NSD2是治疗MASLD的一种有前景的治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Metabolism: clinical and experimental
医学-内分泌学与代谢
CiteScore
18.90
自引率
3.10%
发文量
310
审稿时长
16 days
期刊介绍:
Metabolism upholds research excellence by disseminating high-quality original research, reviews, editorials, and commentaries covering all facets of human metabolism.
Consideration for publication in Metabolism extends to studies in humans, animal, and cellular models, with a particular emphasis on work demonstrating strong translational potential.
The journal addresses a range of topics, including:
- Energy Expenditure and Obesity
- Metabolic Syndrome, Prediabetes, and Diabetes
- Nutrition, Exercise, and the Environment
- Genetics and Genomics, Proteomics, and Metabolomics
- Carbohydrate, Lipid, and Protein Metabolism
- Endocrinology and Hypertension
- Mineral and Bone Metabolism
- Cardiovascular Diseases and Malignancies
- Inflammation in metabolism and immunometabolism
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


