Exploration of dinuclear systems towards catechol oxidase activity with a novel unsymmetric triazole based bis-bidentate ligand
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The design and synthesis of a novel asymmetric triazole-based ligand L1 (N-methyl-3,5-di(pyridin-2-yl)-4H-1,2,4-triazol-4-amine), on which the systematic tuning gave rise to its bisbidentate nature. Corresponding cobalt(II) and nickel(II) complexes ([Co2(L1)2(OH2)4]Cl4; [1](Cl)4 and [Ni2(L1)2(OH2)4]Cl4; [2](Cl)4) were synthesized with axial labile aqua molecules for easier susceptibility towards adduct formation. The arrangement of their molecular framework with robust L1 has been kept planar without the monodentate ligands, which provided reduced steric hindrance for substrate attachment as evident from single crystal X-ray diffraction of [2](Cl)4. 3,5-ditertbutyl catechol was subjected to oxidation in the presence of catalytic amounts of the complexes, and from the spectrophotometric plots, production of the oxidized product (3,5-ditertbutyl quinone) is confirmed. Further kinetic analysis yielded their turnover numbers (kcat values) to be 237 h−1 & 327 h−1 respectively. EPR investigations and mass spectroscopy have been performed, and a plausible mechanism has been established from the fragmented molecular peaks of complex-substrate adducts. This study gives insights into choosing suitable ligands for metal ligand cooperativity.
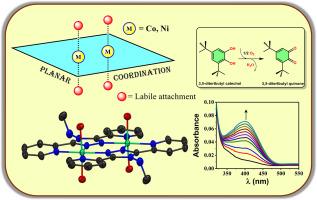
用一种新的不对称三唑基双双齿配体探索双核系统对儿茶酚氧化酶活性的影响
设计合成了一种新型的不对称三唑基配体L1 (n-甲基-3,5-二(吡啶-2-基)- 4h -1,2,4-三唑-4-胺),其系统调谐使其具有双齿性。相应的钴(II)和镍(II)配合物([Co2(L1)2(OH2)4]Cl4;[1](Cl)4和[Ni2(L1)2(OH2)4]Cl4;[2](Cl)4)是用轴向不稳定的水分子合成的,更容易形成加合物。从[2](Cl)4的单晶x射线衍射中可以看出,它们的分子框架在没有单齿配体的情况下保持了平面的排列,从而减少了底物附着的空间位阻。在催化量的配合物存在下,3,5-二叔丁基儿茶酚被氧化,从分光光度图中,证实了氧化产物(3,5-二叔丁基醌)的产生。进一步的动力学分析得出它们的周转数(kcat值)分别为237 h−1和327 h−1。通过EPR研究和质谱分析,从络合物-底物加合物的碎片分子峰中建立了一个合理的机制。该研究为金属配体协同性选择合适的配体提供了新的思路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


