Electrocatalytic proton reduction by a nickel complex with a pentadentate pyridinophane ligand
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Development of efficient and stable molecular catalyst for H2 evolution using earth-abundant materials is one subject of energy research. Inspired by the exceptional performance of [NiFe] hydrogenases in catalyzing H2 evolution under mild pH conditions, the abundant nickel has emerged as a promising candidate for molecular catalysts design. While significant progress has been made in developing Ni-based molecular catalysts with sulfur or phosphine ligands, polypyridine ligand platforms remain much less explored for Ni-based H2 evolution, despite demonstrated great promise of polypyridine ligands in cobalt-based H2 evolution systems. Here, we report a Ni polypyridine complex based on a pentadentate N-methylpyridine-2,11-diaza[3,3]-(2,6)pyridinophane ligand, and evaluate its potential for proton reduction in weakly acidic media, aiming to functionally mimic [NiFe] hydrogenases. Electrochemical studies indicate that it exhibits a high Faradic efficiency of 98 ± 0.97 % for H2 evolution at a modest overpotential of 540 mV when acetic acid is used as proton donor, highlighting its electrocatalytic activity even under weakly acidic conditions. The catalytic rate displays a first-order dependence on acid concentration, with a scan-rate-independent apparent rate constant of 903.58 ± 25.79 M-1·s-1. Unlike most reported Ni-based catalysts that require stronger acids or higher concentrations, this Ni polypyridine complex catalyzes proton reduction efficiently in weakly acidic conditions, representing a notable advancement. Consequently, this work describes a case of a Ni polypyridine molecular HER catalyst that emulates the proton reduction functionality of [NiFe] hydrogenases under mild acidic conditions. This study also expands the scope of polypyridine ligands in nickel catalysis, showcasing their untapped potential for developing stable, cost-effective H2 evolution catalysts with tunable reactivity under mild conditions.
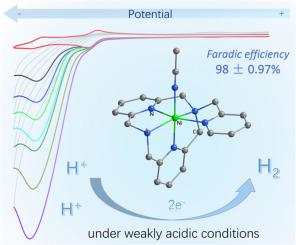
镍配合物与五齿嘧啶配体的电催化质子还原
利用地球上资源丰富的材料开发高效、稳定的氢分子催化剂是能源研究的一个课题。受[NiFe]氢化酶在温和pH条件下催化H2演化的优异性能的启发,丰富的镍已成为分子催化剂设计的有前途的候选物。虽然在开发含硫或膦配体的镍基分子催化剂方面取得了重大进展,但尽管在钴基H2演化体系中展示了多吡啶配体的巨大前景,但对镍基H2演化的多吡啶配体平台的探索仍然很少。本文报道了一种基于五齿齿n -甲基吡啶-2,11-双氮[3,3]-(2,6)吡啶配体的镍多吡啶配合物,并评估了其在弱酸性介质中质子还原的潜力,旨在模拟[NiFe]氢化酶的功能。电化学研究表明,当以乙酸为质子供体时,在540 mV的适度过电位下,其析氢的法氏效率为98±0.97%,即使在弱酸性条件下也具有明显的电催化活性。催化速率与酸浓度呈一级依赖关系,与扫描速率无关的表观速率常数为903.58±25.79 M-1·s-1。与大多数报道的镍基催化剂需要更强的酸或更高的浓度不同,这种镍多吡啶配合物在弱酸性条件下有效地催化质子还原,这是一个显著的进步。因此,本研究描述了一种镍多吡啶分子HER催化剂,它在温和的酸性条件下模拟了[NiFe]氢化酶的质子还原功能。该研究还扩大了多吡啶配体在镍催化中的应用范围,展示了它们在开发稳定、经济、温和条件下反应活性可调的析氢催化剂方面尚未开发的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


