Size-dependent encapsulation selectivity of alkaline earth metal ions in distorted octahedral and dicapped Ni(II) metallacryptate with L-isoleucine
IF 4.7
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
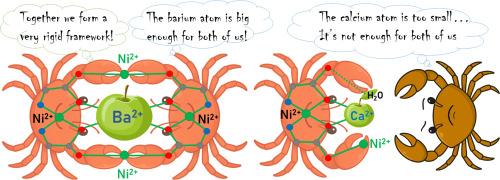
The study of size selectivity of cryptands and crown ethers towards metal ions is one of the most interesting and promising directions in supramolecular chemistry. In this work the metallacryptates [MNi6(Ile)12](ClO4)2 (M = Ba2+, Sr2+; Ile = l-isoleucine) (2–3) and the complex [Ca2Ni6(Ile)12(CH3OH)6(H2O)3](ClO4)4 (4) were synthesized for the first time. The composition and structure of the obtained compounds were studied by single-crystal X-ray diffraction (SCXRD), IR-spectroscopy, CHN analysis, electrospray ionization mass spectrometry (ESI-MS) and energy-dispersive X-ray spectroscopy (EDX). The time-dependent dynamics of self-assembly of metallacryptates 2–3 was investigated by PCA method. The intermediate complex ion [MNi5(Ile)10]2+ was discovered, it coexists in solution together with complexes 2–3. The stability constants of the complexes [MNi6(Ile)12]2+ decrease in the series Ba2+ – Sr2+. It is shown that the class of octahedral Ni(II) metallacryptates with amino acids possesses a rigid [Ni6(AA)12] framework (AA = amino acids) and exhibits very high size and charge selectivity towards the central atom, showing no binding affinity for monovalent cations and Mg2+. Significant changes in the crystal radius across the series of alkaline earth metal ions (M2+) allowed us to investigate the size selectivity of octahedral M(II)-Ni(II) metallacryptates with l-isoleucine. Complexes 2–3 belong to the class of octahedral metallacryptates. The possibility of the existence of 4 was previously denied in the literature. Changes in structural parameters correlate well with the electronic structure, investigated by quantum chemical calculations and UV–Vis spectroscopy within the ligand field theory.
碱土金属离子在扭曲八面体和l -异亮氨酸残基镍金属酸盐中的包封选择性
研究隐键和冠醚对金属离子的尺寸选择性是超分子化学中最有趣和最有前途的方向之一。本文首次合成了金属螯合物[MNi6(Ile)12](ClO4)2 (M = Ba2+, Sr2+; Ile = l-异亮氨酸)(2 - 3)和配合物[Ca2Ni6(Ile)12(CH3OH)6(H2O)3](ClO4)4(4)。采用单晶x射线衍射(SCXRD)、红外光谱(ir)、CHN分析、电喷雾电离质谱(ESI-MS)和能量色散x射线能谱(EDX)等方法对所得化合物的组成和结构进行了研究。采用主成分分析法研究了金属酸盐2-3自组装的时间动力学。发现中间络合物离子[MNi5(Ile)10]2+与配合物2 - 3共存于溶液中。配合物[MNi6(Ile)12]2+的稳定性常数在Ba2+ - Sr2+系列中降低。结果表明,氨基酸型八面体Ni(II)金属酸盐具有刚性的[Ni6(AA)12]骨架(AA =氨基酸),对中心原子具有很高的尺寸和电荷选择性,对单价阳离子和Mg2+没有结合亲和力。在碱土金属离子(M2+)系列中,晶体半径的显著变化使我们能够研究l-异亮氨酸对八面体M(II)-Ni(II)金属酸盐的尺寸选择性。配合物2-3属于八面体金属酸盐类。先前文献中否认了4存在的可能性。通过量子化学计算和紫外可见光谱在配体场理论中研究了结构参数的变化与电子结构的关系。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


