Transient increase in skeletal-related events after discontinuation of high-dose denosumab in cancer patients
IF 3.5
2区 医学
Q2 Medicine
引用次数: 0
Abstract
Background
Denosumab is widely used to prevent skeletal-related events (SREs) in patients with bone metastases. However, rebound bone resorption after discontinuation is recognized. In osteoporosis, discontinuation of low-dose denosumab increases multiple vertebral fractures, but data on high-dose discontinuation remain limited.
Methods
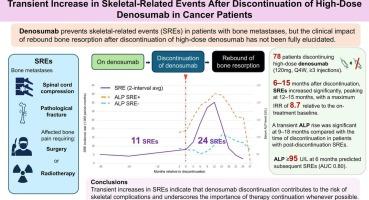
Among 493 patients treated with high-dose denosumab at our institution (2014–2023), 78 met eligibility criteria. SREs during and after treatment were compared using each patient as their own control. SREs were defined as pathological fracture, spinal cord compression, or radiotherapy/surgery for metastatic bone pain. Hypercalcemia, benign fragility fractures, and serum ALP level were also assessed.
Results
A total of 11 SREs were observed during denosumab and 24 after discontinuation. Post-discontinuation incidence was 14.1 per 1,000 person-months, 3.3 times higher than during treatment (95 % CI, 1.4–7.8). The increase was significant at 6–15 months, peaking at 12–15 months (IRR 8.7, 95 % CI, 2.8–27.5), and declined thereafter. Fewer denosumab doses were also associated with a higher risk of SREs after discontinuation. Two benign fragility fractures occurred during denosumab and four after discontinuation. Grade ≥ 3 hypercalcemia occurred only after discontinuation (3 cases). Transient ALP elevation at 9–18 months was observed in patients with post-discontinuation SREs, and ALP ≥ 95 U/L at 6 months predicted subsequent SREs (AUC 0.80).
Conclusion
SREs increased significantly 6–15 months after high-dose denosumab discontinuation. Elevated ALP was associated with post-discontinuation SREs. These findings emphasize that discontinuation contributes to SRE risk, possibly via rebound bone resorption, and underscore the importance of continuation of therapy whenever possible.
癌症患者停用高剂量地诺单抗后骨骼相关事件的短暂增加
背景:denosumab被广泛用于预防骨转移患者的骨骼相关事件(SREs)。然而,停药后反弹骨吸收是公认的。在骨质疏松症中,停用低剂量地诺单抗会增加多发性椎体骨折,但停用高剂量地诺单抗的数据仍然有限。方法在我院(2014-2023)493例接受大剂量地诺单抗治疗的患者中,78例符合入选标准。以每位患者为对照,比较治疗期间和治疗后的SREs。SREs被定义为病理性骨折、脊髓压迫或转移性骨痛的放疗/手术。同时评估高钙血症、良性脆性骨折和血清ALP水平。结果在地诺单抗治疗期间共观察到11例SREs,停药后共观察到24例SREs。停药后的发病率为14.1 / 1000人月,是治疗期间的3.3倍(95% CI, 1.4-7.8)。6-15个月时增加显著,12-15个月时达到峰值(IRR 8.7, 95% CI 2.8-27.5),此后下降。较少的地诺单抗剂量也与停药后SREs的高风险相关。在denosumab期间发生2例良性脆性骨折,停药后发生4例。≥3级高钙血症仅在停药后发生(3例)。停药后SREs患者在9-18个月时观察到一过性ALP升高,6个月时ALP≥95 U/L预测后续SREs (AUC 0.80)。结论大剂量地诺单抗停药后6 ~ 15个月sres显著升高。ALP升高与停药后SREs相关。这些发现强调了停药可能通过反弹骨吸收增加SRE风险,并强调了尽可能继续治疗的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Bone Oncology
ONCOLOGY-
CiteScore
7.20
自引率
2.90%
发文量
50
审稿时长
34 days
期刊介绍:
The Journal of Bone Oncology is a peer-reviewed international journal aimed at presenting basic, translational and clinical high-quality research related to bone and cancer.
As the first journal dedicated to cancer induced bone diseases, JBO welcomes original research articles, review articles, editorials and opinion pieces. Case reports will only be considered in exceptional circumstances and only when accompanied by a comprehensive review of the subject.
The areas covered by the journal include:
Bone metastases (pathophysiology, epidemiology, diagnostics, clinical features, prevention, treatment)
Preclinical models of metastasis
Bone microenvironment in cancer (stem cell, bone cell and cancer interactions)
Bone targeted therapy (pharmacology, therapeutic targets, drug development, clinical trials, side-effects, outcome research, health economics)
Cancer treatment induced bone loss (epidemiology, pathophysiology, prevention and management)
Bone imaging (clinical and animal, skeletal interventional radiology)
Bone biomarkers (clinical and translational applications)
Radiotherapy and radio-isotopes
Skeletal complications
Bone pain (mechanisms and management)
Orthopaedic cancer surgery
Primary bone tumours
Clinical guidelines
Multidisciplinary care
Keywords: bisphosphonate, bone, breast cancer, cancer, CTIBL, denosumab, metastasis, myeloma, osteoblast, osteoclast, osteooncology, osteo-oncology, prostate cancer, skeleton, tumour.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


