A 3D UNet-based fusion network for brain tumor segmentation with missing modalities
IF 6.5
2区 计算机科学
Q1 COMPUTER SCIENCE, ARTIFICIAL INTELLIGENCE
引用次数: 0
Abstract
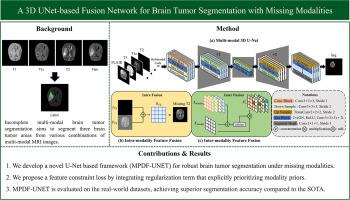
Multimodal magnetic resonance imaging provides complementary information for brain tumor segmentation, significantly enhancing the accuracy of diagnosis and prognosis. However, the common issue of missing modalities in clinical practice severely undermines the performance of existing methods, as they predominantly rely on complete multimodal data and struggle to effectively handle dynamic inter-modality correlations and tumor region specificity. To address this challenge, we propose a novel fusion network based on 3D U-Net, termed MPDF-UNET. Its core innovation lies in the introduction of the Modality Priors and Dynamic Features fusion (MPDF) module, which adaptively learns the unique representations of different MRI modalities under conditions of partial modality loss while effectively integrating complementary information across modalities. Additionally, we develop a modality combination sampling strategy that dynamically adjusts the distribution of modality combinations in the training data. This strategy encourages the network to fully exploit prior knowledge from each modality, thereby enhancing model robustness under conditions of missing modalities. To mitigate the impact of missing modality-associated dynamic feature information, we further propose a feature loss function. By imposing constraints on dynamic features, this loss function facilitates the learning of modality priors, alleviating the degradation of the network’s representational capacity caused by missing modalities. Experiments conducted on BRATS2018 and BRATS2020 benchmark datasets demonstrate the superiority of MPDF-UNET. Notably, the model achieves significant improvements in the fine-grained segmentation of enhancing tumors, surpassing current SOTA. Specifically, on BRATS2018 dataset, our method improves the Dice score of enhancing tumor segmentation by 7.78 % on average compared to the best-performing baseline Region-aware Fusion Network (RFNet), demonstrating superior robustness under missing modalities. This work provides a reliable solution for incomplete or resource-limited multimodal data in clinical settings, demonstrating significant practical value.
一种基于unet的三维融合网络用于缺失模态的脑肿瘤分割
多模态磁共振成像为脑肿瘤分割提供了补充信息,显著提高了诊断和预后的准确性。然而,临床实践中常见的模式缺失问题严重影响了现有方法的性能,因为它们主要依赖于完整的多模式数据,难以有效处理动态的模式间相关性和肿瘤区域特异性。为了应对这一挑战,我们提出了一种基于3D U-Net的新型融合网络,称为MPDF-UNET。其核心创新在于引入了模态先验和动态特征融合(MPDF)模块,该模块在部分模态丢失的情况下自适应学习不同MRI模态的独特表示,同时有效地整合了模态之间的互补信息。此外,我们开发了一种模态组合采样策略,可以动态调整训练数据中模态组合的分布。该策略鼓励网络充分利用每个模态的先验知识,从而增强模型在缺失模态条件下的鲁棒性。为了减轻模态相关动态特征信息缺失的影响,我们进一步提出了特征损失函数。通过对动态特征施加约束,该损失函数促进了模态先验的学习,减轻了模态缺失导致的网络表征能力下降。在BRATS2018和BRATS2020基准数据集上进行的实验证明了MPDF-UNET的优越性。值得注意的是,该模型在增强肿瘤的细粒度分割方面取得了显著的改进,超过了目前的SOTA。具体而言,在BRATS2018数据集上,与表现最好的基线区域感知融合网络(RFNet)相比,我们的方法将增强肿瘤分割的Dice评分平均提高了7.78%,显示出在缺失模式下的优越鲁棒性。这项工作为临床环境中不完整或资源有限的多模态数据提供了可靠的解决方案,具有重要的实用价值。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Neurocomputing
工程技术-计算机:人工智能
CiteScore
13.10
自引率
10.00%
发文量
1382
审稿时长
70 days
期刊介绍:
Neurocomputing publishes articles describing recent fundamental contributions in the field of neurocomputing. Neurocomputing theory, practice and applications are the essential topics being covered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


