Experimental measurement and thermodynamic modeling for the methane + methanol binary system: volumetric behavior and Joule-Thomson coefficient modeling
IF 2.7
3区 工程技术
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
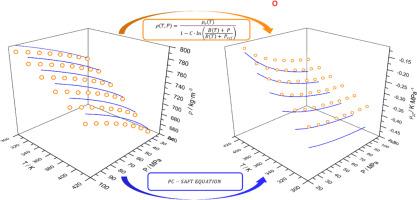
To address the critical role of the Joule-Thomson (JT) effect in flow assurance for the oil and gas industry, this work presents experimental density data for methane + methanol mixtures at temperatures ranging from 313.15 K to 413.15 K and pressures up to 100 MPa. Using this data, derivative properties such as isothermal compressibility, isobaric expansivity, and Joule-Thomson coefficient were calculated via a correlative Tammann-Tait Eq. and compared against predictions using the PC-SAFT Eq. of state. The results showed that was negative under these conditions, indicating a warming effect upon expansion. The use of the PC-SAFT Eq. was satisfactory, given the system’s complexity, for density data and for properties that did not rely on the isobaric heat capacity. Additionally, a new binary interaction parameter for the methane/methanol pair was obtained and evaluated using the PC-SAFT Eq. of state.
甲烷+甲醇二元体系的实验测量和热力学建模:体积行为和焦耳-汤姆逊系数建模
为了解决焦耳-汤姆逊(JT)效应在油气行业流动保障中的关键作用,本研究提供了甲烷+甲醇混合物在温度范围为313.15 K至413.15 K,压力高达100 MPa时的实验密度数据。利用这些数据,通过相关的Tammann-Tait方程计算了等温压缩率、等压膨胀率和焦耳-汤姆森系数等导数性质,并与PC-SAFT状态方程的预测结果进行了比较。结果表明,在此条件下μJT为负,说明膨胀过程中存在升温效应。考虑到系统的复杂性,对于密度数据和不依赖于等压热容的性质,PC-SAFT方程的使用令人满意。此外,利用PC-SAFT状态方程,得到了甲烷/甲醇二元相互作用的新参数。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fluid Phase Equilibria
工程技术-工程:化工
CiteScore
5.30
自引率
15.40%
发文量
223
审稿时长
53 days
期刊介绍:
Fluid Phase Equilibria publishes high-quality papers dealing with experimental, theoretical, and applied research related to equilibrium and transport properties of fluids, solids, and interfaces. Subjects of interest include physical/phase and chemical equilibria; equilibrium and nonequilibrium thermophysical properties; fundamental thermodynamic relations; and stability. The systems central to the journal include pure substances and mixtures of organic and inorganic materials, including polymers, biochemicals, and surfactants with sufficient characterization of composition and purity for the results to be reproduced. Alloys are of interest only when thermodynamic studies are included, purely material studies will not be considered. In all cases, authors are expected to provide physical or chemical interpretations of the results.
Experimental research can include measurements under all conditions of temperature, pressure, and composition, including critical and supercritical. Measurements are to be associated with systems and conditions of fundamental or applied interest, and may not be only a collection of routine data, such as physical property or solubility measurements at limited pressures and temperatures close to ambient, or surfactant studies focussed strictly on micellisation or micelle structure. Papers reporting common data must be accompanied by new physical insights and/or contemporary or new theory or techniques.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


