The solubility of O-methylphenylacetic acid (OMPA) in different pure solvents, corrections and thermodynamic properties
IF 2.7
3区 工程技术
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
This study investigated the dissolution behavior of O-methylphenylacetic acid (OMPA) in twelve organic solvent systems (methanol, ethanol, n-propanol, i-propanol, n-butanol, i-butanol, acetone, acetonitrile, dichloromethane, 1,2-dichloroethane, methyl acetate, ethyl acetate) across eight temperature gradients. The solubility of all tested solvents increased with temperature, and acetone exhibited the highest solubility among them. A multidimensional research approach was employed to elucidate the mechanisms underlying the dissolution process. This approach integrated molecular electrostatic potential surface (MEPS) analysis, the interpretation of solvent physicochemical parameters, and density functional theory (DFT) calculations. Six thermodynamic models (λh, modified Apelblat, van't Hoff, Yaws, Wilson, and Jouyban models) were applied to correlate the regularity of solubility evolution. The validity of these models was evaluated through ARD and RMSD. Among these models examined, the Yaws model demonstrated optimal fitting performance with a 100ARD average of 0.8221. Additionally, thermodynamic analysis revealed patterns concerning changes in the apparent mixed Gibbs free energy (ΔsolG), the apparent mixing enthalpy change (ΔsolH), and the apparent mixing entropy change (ΔsolS) throughout the dissolution process. It was observed that the dissolution of OMPA is endothermic and driven by an increase in entropy.
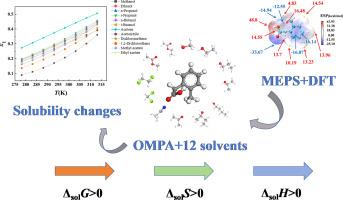
邻甲基苯基乙酸(OMPA)在不同纯溶剂中的溶解度、修正和热力学性质
研究了邻甲基苯基乙酸(OMPA)在12种有机溶剂体系(甲醇、乙醇、正丙醇、正丙醇、正丁醇、正丁醇、正丁醇、丙酮、乙腈、二氯甲烷、1,2-二氯乙烷、乙酸甲酯、乙酸乙酯)中8个温度梯度下的溶解行为。溶剂的溶解度随温度升高而升高,其中丙酮的溶解度最高。采用多维研究方法来阐明溶解过程的机制。该方法集成了分子静电电位表面(MEPS)分析、溶剂理化参数解释和密度泛函理论(DFT)计算。采用λh、修正Apelblat、van't Hoff、Yaws、Wilson和Jouyban模型分析了溶解度演化规律。通过ARD和RMSD评价模型的有效性。其中,雅司模型拟合效果最佳,100ARD平均值为0.8221。此外,热力学分析揭示了溶解过程中表观混合吉布斯自由能(ΔsolG)、表观混合焓变(ΔsolH)和表观混合熵变(ΔsolS)的变化规律。观察到OMPA的溶解是吸热的,由熵的增加驱动。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fluid Phase Equilibria
工程技术-工程:化工
CiteScore
5.30
自引率
15.40%
发文量
223
审稿时长
53 days
期刊介绍:
Fluid Phase Equilibria publishes high-quality papers dealing with experimental, theoretical, and applied research related to equilibrium and transport properties of fluids, solids, and interfaces. Subjects of interest include physical/phase and chemical equilibria; equilibrium and nonequilibrium thermophysical properties; fundamental thermodynamic relations; and stability. The systems central to the journal include pure substances and mixtures of organic and inorganic materials, including polymers, biochemicals, and surfactants with sufficient characterization of composition and purity for the results to be reproduced. Alloys are of interest only when thermodynamic studies are included, purely material studies will not be considered. In all cases, authors are expected to provide physical or chemical interpretations of the results.
Experimental research can include measurements under all conditions of temperature, pressure, and composition, including critical and supercritical. Measurements are to be associated with systems and conditions of fundamental or applied interest, and may not be only a collection of routine data, such as physical property or solubility measurements at limited pressures and temperatures close to ambient, or surfactant studies focussed strictly on micellisation or micelle structure. Papers reporting common data must be accompanied by new physical insights and/or contemporary or new theory or techniques.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


