Measurements of infinite dilution binary diffusion coefficient and partial molar volume of piperine in supercritical carbon dioxide
IF 2.7
3区 工程技术
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
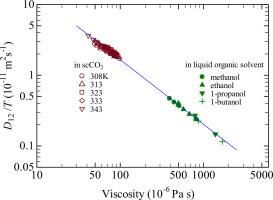
Piperine is one of key components of peppers, and supercritical fluid extraction has been applied to isolate such heat-sensitive natural substances. The diffusion coefficient (D12) of piperine in supercritical (sc) carbon dioxide is essential for the process design, but no data are available in the literature. In the present study the infinite dilution binary diffusion coefficient (D12) of piperine in scCO2 was measured at temperatures from 308.2 K to 343.2 K and at pressures from 12 MPa to 30 MPa by the chromatographic impulse response method, and those in methanol, ethanol, 1-propanol and 1-butanol were measured at atmospheric pressure and at temperatures from 303.2 K to 343.2 K by the Taylor dispersion method. The hydrodynamic equation, D12/T =αηβ, T is the temperature and η is the CO2 and alcohol viscosity, was found to well represent all of the D12 values measured in both scCO2 and atmospheric liquid alcohols, with a single set of two constants α = 3.655 × 10–15 kg-βmβ+2sβ-1K-1 and β = -0.9145 with the average absolute relative deviation of 4.41 % and maximum deviation of 14.0 % with 72 measurement conditions. Partial molar volumes(PMV) of piperine determined from the retention factors were negative and decreased substantially closer to the CO2 critical point, as observed for various solutes in scCO2 reported in the literature. To evaluate the accuracy of the determined PMV values new reliable models and reliable solubility data are needed to describe the PMV values, especially near the critical region.
超临界二氧化碳中胡椒碱无限稀释、二元扩散系数和部分摩尔体积的测量
胡椒碱是辣椒的关键成分之一,超临界流体萃取法已被用于分离这种热敏性天然物质。胡椒碱在超临界(sc)二氧化碳中的扩散系数(D12)对工艺设计至关重要,但文献中没有相关数据。用色谱脉冲响应法测定了胡椒碱在scCO2中的无限稀释二元扩散系数(D12),温度为308.2 K ~ 343.2 K,压力为12 MPa ~ 30 MPa,在常压和温度为303.2 K ~ 343.2 K时,用Taylor色散法测定了胡椒碱在甲醇、乙醇、1-丙醇和1-丁醇中的无限稀释二元扩散系数(D12)。在72种测量条件下,流体动力学方程D12/T =αηβ, T为温度,η为CO2和醇粘度,可以很好地代表scCO2和常压液体醇的所有D12值,其中α = 3.655 × 10-15 kg-βm -β +2s -β - 1k -1和β = -0.9145,平均绝对相对偏差为4.41%,最大偏差为14.0%。根据保留系数测定的胡椒碱的偏摩尔体积(PMV)为负,并且在接近CO2临界点时大幅下降,这与文献中报道的scCO2中各种溶质的观察结果一致。为了评估所确定的PMV值的准确性,需要新的可靠模型和可靠的溶解度数据来描述PMV值,特别是在临界区域附近。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Fluid Phase Equilibria
工程技术-工程:化工
CiteScore
5.30
自引率
15.40%
发文量
223
审稿时长
53 days
期刊介绍:
Fluid Phase Equilibria publishes high-quality papers dealing with experimental, theoretical, and applied research related to equilibrium and transport properties of fluids, solids, and interfaces. Subjects of interest include physical/phase and chemical equilibria; equilibrium and nonequilibrium thermophysical properties; fundamental thermodynamic relations; and stability. The systems central to the journal include pure substances and mixtures of organic and inorganic materials, including polymers, biochemicals, and surfactants with sufficient characterization of composition and purity for the results to be reproduced. Alloys are of interest only when thermodynamic studies are included, purely material studies will not be considered. In all cases, authors are expected to provide physical or chemical interpretations of the results.
Experimental research can include measurements under all conditions of temperature, pressure, and composition, including critical and supercritical. Measurements are to be associated with systems and conditions of fundamental or applied interest, and may not be only a collection of routine data, such as physical property or solubility measurements at limited pressures and temperatures close to ambient, or surfactant studies focussed strictly on micellisation or micelle structure. Papers reporting common data must be accompanied by new physical insights and/or contemporary or new theory or techniques.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


