Identification of isatin-triazole-benzenesulfonamide hybrids as dual hCA IX/XII and c-met inhibitors with hypoxia-mediated chemo-sensitizing activity
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
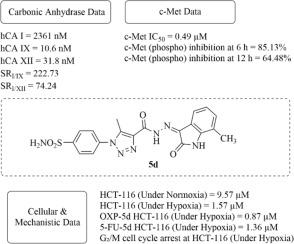
One of the main factors contributing to treatment resistance and a poor prognosis in colorectal cancer is hypoxia. Eleven isatin-based hybrids comprising 1,2,3-triazole and benzenesulfonamide fragments (5a-f and 7a-e) were logically designed and synthesized in this study to investigate their dual inhibitory potential against carbonic anhydrases IX and XII (CA IX/XII) and receptor tyrosine kinase c-Met, two hypoxia-related targets. Strong nanomolar inhibition of CA IX/XII and sub-micromolar to low-micromolar inhibition of c-Met were shown by a number of drugs (5c, 5d, 5e, 5f, and 7e). Compound 5d had the highest activity (IC₅₀ = 1.57 μM under hypoxia vs. 9.57 μM under normoxia), according to a subsequent cytotoxicity assay in HCT-116 colorectal cancer cells, which showed improved potency of all lead compounds under hypoxic conditions. In the cell lines HT-29 and SW-620, the better profile of 5d was further validated. Mechanistic investigations revealed that 5d triggered apoptosis and caused G₂/M phase arrest, confirming its function in hypoxia-driven cytotoxicity. Additionally, in hypoxic conditions, 5d significantly increased the effectiveness of 5-fluorouracil (5-FU) and oxaliplatin (OXP), increasing its potency by more than 10 times. Positive pharmacokinetic and drug-like characteristics were validated through in silico ADME profiling. Molecular docking investigations revealed that 5d exhibited strong binding interactions within the c-Met and CA IX/XII active sites. Compound 5d could be a promising dual-targeting option for overcoming hypoxia-associated resistance in colorectal cancer, as indicated by these data.
isatin-triazol - benesulfonamide杂交种作为双hCA IX/XII和c-met抑制剂的鉴定,具有缺氧介导的化学增敏活性
导致结直肠癌治疗抵抗和预后不良的主要因素之一是缺氧。本研究逻辑地设计并合成了11个isatin-based杂合体,包括1,2,3-三唑和苯磺酰胺片段(5a-f和7a-e),以研究它们对碳酸酐酶IX和XII (CA IX/XII)和受体酪氨酸激酶c-Met(两个缺氧相关靶点)的双重抑制潜力。许多药物(5c, 5d, 5e, 5f和7e)显示出对CA IX/XII的强纳摩尔抑制和对c-Met的亚微摩尔至低微摩尔抑制。根据随后在HCT-116结肠直肠癌细胞中进行的细胞毒性试验,化合物5d具有最高的活性(IC₅0 = 1.57 μM在缺氧下对9.57 μM在常氧下),该试验显示所有先导化合物在缺氧条件下的效力都有所提高。在HT-29和SW-620细胞系中,进一步验证了5d较好的表达谱。机制研究表明5d触发细胞凋亡并引起G₂/M相阻滞,证实了其在缺氧驱动的细胞毒性中的作用。此外,在低氧条件下,5d显著提高了5-氟尿嘧啶(5-FU)和奥沙利铂(OXP)的有效性,其效力提高了10倍以上。通过计算机ADME分析验证了阳性药代动力学和药物样特征。分子对接研究表明,5d在c-Met和CA IX/XII活性位点表现出很强的结合相互作用。这些数据表明,化合物5d可能是克服结直肠癌缺氧相关耐药的有希望的双靶向选择。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


