Binding and ubiquitination-mediated degradation of ACKR3 by the novel Scutellarein derivative TBS6b potently suppresses hepatocellular carcinoma
IF 4.7
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Scutellarein, a flavonoid compound from the traditional Chinese herb Scutellaria baicalensis, exhibits inhibitory effects against hepatocellular carcinoma (HCC), but its clinical application is limited by relatively weak potency. To enhance its antitumor activity, we synthesized a novel derivative, 5,6,7-trimethoxy-4′-benzimidazolyl scutellarein 6b (TBS6b), by introducing antitumor pharmacophores—trimethoxyphenyl and benzimidazole—into the scutellarein scaffold. TBS6b demonstrated significantly improved anti-HCC activity both in vitro and in vivo. Cell-based assays, including colony formation, EdU staining, wound healing, transwell migration, and western blot analysis, demonstrated that TBS6b significantly inhibits HCC cell proliferation, migration, and invasion. Mechanistically, we employed proteomic and transcriptomic sequencing, along with western blot and qRT-PCR experiments, to predict and validate atypical chemokine receptor 3 (ACKR3) as the target of TBS6b. Molecular docking studies confirmed that TBS6b binds tightly to the ACKR3 protein. Additionally, with the aid of pharmacological tools, we established that TBS6b promotes the ubiquitination and degradation of ACKR3. Tissue microarray analysis and queries of public databases revealed that ACKR3 expression is elevated in HCC tissues compared to adjacent non-cancerous tissues, correlating closely with patient survival. By constructing cell lines with either silenced or overexpressed ACKR3, we confirmed that ACKR3 promotes the proliferation, migration, and invasion of HCC. Finally, rescue experiments indicated that TBS6b exerts its anticancer effects primarily through targeting ACKR3. These findings establish ACKR3 as a critical target through which TBS6b mediates its anticancer activity against HCC.
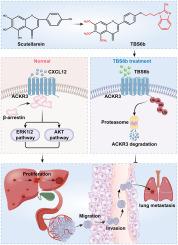
新型灯盏花苷衍生物TBS6b结合和泛素化介导的ACKR3降解可有效抑制肝细胞癌
黄芩苷是中药黄芩中的一种黄酮类化合物,对肝癌具有抑制作用,但效力较弱,限制了其临床应用。为了增强其抗肿瘤活性,我们将抗肿瘤药物载体三甲氧基苯基和苯并咪唑引入黄芩苷支架中,合成了新的衍生物5,6,7-三甲氧基-4 ' -苯并咪唑-黄芩苷6b (TBS6b)。TBS6b在体外和体内均显示出显著提高的抗hcc活性。基于细胞的实验,包括菌落形成、EdU染色、伤口愈合、跨井迁移和western blot分析,表明TBS6b显著抑制HCC细胞的增殖、迁移和侵袭。在机制上,我们采用蛋白质组学和转录组学测序,以及western blot和qRT-PCR实验来预测和验证非典型趋化因子受体3 (ACKR3)是TBS6b的靶标。分子对接研究证实TBS6b与ACKR3蛋白紧密结合。此外,借助药理学工具,我们确定TBS6b促进ACKR3的泛素化和降解。组织微阵列分析和公共数据库查询显示,与邻近非癌组织相比,HCC组织中ACKR3表达升高,与患者生存密切相关。通过构建ACKR3沉默或过表达的细胞系,我们证实ACKR3促进HCC的增殖、迁移和侵袭。最后,救援实验表明TBS6b主要通过靶向ACKR3发挥其抗癌作用。这些发现证实ACKR3是TBS6b介导其抗肝癌活性的关键靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Bioorganic Chemistry
生物-生化与分子生物学
CiteScore
9.70
自引率
3.90%
发文量
679
审稿时长
31 days
期刊介绍:
Bioorganic Chemistry publishes research that addresses biological questions at the molecular level, using organic chemistry and principles of physical organic chemistry. The scope of the journal covers a range of topics at the organic chemistry-biology interface, including: enzyme catalysis, biotransformation and enzyme inhibition; nucleic acids chemistry; medicinal chemistry; natural product chemistry, natural product synthesis and natural product biosynthesis; antimicrobial agents; lipid and peptide chemistry; biophysical chemistry; biological probes; bio-orthogonal chemistry and biomimetic chemistry.
For manuscripts dealing with synthetic bioactive compounds, the Journal requires that the molecular target of the compounds described must be known, and must be demonstrated experimentally in the manuscript. For studies involving natural products, if the molecular target is unknown, some data beyond simple cell-based toxicity studies to provide insight into the mechanism of action is required. Studies supported by molecular docking are welcome, but must be supported by experimental data. The Journal does not consider manuscripts that are purely theoretical or computational in nature.
The Journal publishes regular articles, short communications and reviews. Reviews are normally invited by Editors or Editorial Board members. Authors of unsolicited reviews should first contact an Editor or Editorial Board member to determine whether the proposed article is within the scope of the Journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


