Metal-organic frameworks (MOFs)-based on ZIFs for glyphosate remediation in aqueous effluent: synthesis, characterization, and adsorptive performance
IF 5.2
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Glyphosate is the most widely used herbicide worldwide. This non-selective, water-soluble organophosphorus compound can reach surface and groundwater due to its soil mobility, contributing to aquatic contamination. Chronic exposure has been linked to adverse human health effects. These concerns have increased interest in remediation techniques, with adsorption standing out for its efficiency, simplicity, and low cost. Among adsorbents, metal-organic frameworks (MOFs), particularly zeolitic imidazolate frameworks (ZIFs), are of great interest due to their high surface areas, porosity, and thermal and chemical stability. In this study, Cu, Co, and Ni-based ZIFs were synthesized using 2-methylimidazole as the ligand, to apply them in the adsorption of the herbicide glyphosate from aqueous media. Adsorption experiments showed a low influence of pH and equilibrium was reached within 8 h. The kinetics followed a pseudo-second-order model, suggesting a predominant chemisorption mechanism. The isotherms fitted well to the Langmuir model, with maximum adsorption capacities (Qmax) of 33.99, 58.49, and 64.73 mg·g−1 for CuZIF, CoZIF, and NiZIF, respectively. The differences in performance were attributed to the electronic and structural properties of the metal ions, which influence the affinity toward the functional groups of glyphosate and the formation of coordination bonds. The results are comparable to those reported for widely studied MOFs, with the advantage that the synthesized materials present simple, cost-effective, and environmentally friendly synthesis routes. Therefore, the developed ZIFs demonstrate high potential for application in the remediation of glyphosate in aqueous effluents.
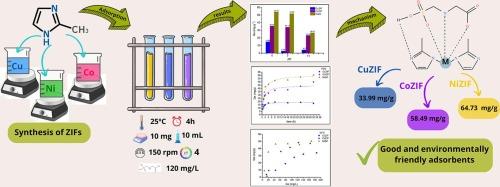
基于ZIFs的金属有机骨架(MOFs)用于水处理废水中草甘膦的修复:合成、表征和吸附性能
草甘膦是世界上使用最广泛的除草剂。这种非选择性的水溶性有机磷化合物由于其土壤流动性可以到达地表水和地下水,造成水生污染。长期接触会对人体健康产生不利影响。这些问题增加了人们对修复技术的兴趣,吸附因其效率、简单性和低成本而脱颖而出。在吸附剂中,金属有机骨架(MOFs),特别是沸石咪唑酸骨架(ZIFs),由于其高表面积、孔隙度、热稳定性和化学稳定性而备受关注。本研究以2-甲基咪唑为配体合成了Cu、Co和ni基zif,并将其应用于对草甘膦除草剂的吸附。吸附实验结果表明,pH对吸附的影响较小,在8 h内达到吸附平衡。吸附动力学符合准二级模型,表明主要的吸附机理为化学吸附。CuZIF、CoZIF和NiZIF的最大吸附量(Qmax)分别为33.99、58.49和64.73 mg·g−1,符合Langmuir模型。金属离子的电子和结构特性影响了对草甘膦官能团的亲和力和配位键的形成。结果与广泛研究的mof相媲美,其优点是合成材料具有简单,经济,环保的合成路线。因此,所开发的zif在水处理草甘膦方面具有很大的应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


