Modulate surface potential well depth of Bi12O17Cl2 by FeOOH in Bi12O17Cl2@FeOOH heterojunction to boost piezoelectric charge transfer and piezo-self-Fenton catalysis
IF 13.5
2区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Although the design of heterojunction piezoelectric catalysts has significantly enhanced catalytic activity, the regulatory mechanisms of heterojunction interfaces on surface potential wells during piezoelectric processes and their impact on carrier migration still lack systematic investigation. This work constructs an enhance interface interaction heterointerface between amorphous FeOOH and Bi12O17Cl2 (BOC) in Bi12O17Cl2@FeOOH through a self-assembly strategy. This strong interfacial interaction significantly enhances interface polarity can substantially suppress the stress-responsive capability of surface charges on BOC (maximum reduction reached as high as 63 %–98 % of original value). This significantly reduces the depth of surface potential wells during piezoelectric processes, thereby effectively weakening piezoelectric charge confinement while promoting charge transfer. Concurrently, Bi–O–Fe chemical bonds formed at the interface and establish charge transport channels. These synergistic mechanisms elevate the H2O2 production rate to 3.04 mmol g−1 h−1 for participate in the piezoelectric self-Fenton reaction and the removal rate of total organic carbon increased 3 fold (18.6 % vs 55.8 %).
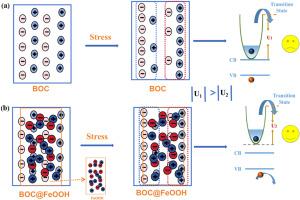
FeOOH在Bi12O17Cl2@FeOOH异质结中调制Bi12O17Cl2表面电位井深,促进压电电荷转移和压电自fenton催化
虽然异质结压电催化剂的设计显著提高了催化活性,但在压电过程中异质结界面对表面电位阱的调控机制及其对载流子迁移的影响仍缺乏系统的研究。本工作通过自组装策略在Bi12O17Cl2@FeOOH中构建了非晶FeOOH与Bi12O17Cl2 (BOC)之间增强界面相互作用的异质界面。这种强的界面相互作用显著增强了界面极性,大大抑制了BOC表面电荷的应力响应能力(最大降幅可达原值的63% - 98%)。这大大降低了压电过程中表面电位阱的深度,从而有效地削弱了压电电荷约束,同时促进了电荷转移。同时,在界面处形成Bi-O-Fe化学键,建立电荷传输通道。这些协同机制使参与压电自fenton反应的H2O2产率提高到3.04 mmol g−1 h−1,总有机碳去除率提高了3倍(18.6%比55.8%)。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


