Pulmonary inflammation and immune responses induced by nanocellulose: Insights from in vivo and in vitro models
IF 2.9
Q2 TOXICOLOGY
引用次数: 0
Abstract
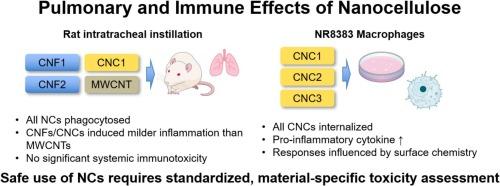
The increasing use of nanocellulose (NC), including cellulose nanofibrils (CNFs) and cellulose nanocrystals (CNCs), in industrial, biomedical, and consumer products has raised concerns regarding potential inhalation exposure, as these materials contain components within the respirable particle size range (<10 µm). Despite expanding applications, data on NC-induced pulmonary and systemic immune effects remain limited. This study investigated the pulmonary and immunotoxic effects of CNF1 (TEMPO-oxidized), CNF2 (mechanically fibrillated), and CNC1 in rats following intratracheal instillation, using multi-walled carbon nanotubes (MWCNTs) as a benchmark. All materials were administered at 2.0 mg/kg body weight, with fiber diameters of 14.1–28.2 nm and lengths of 0.7–2.2 µm. At 28 d post-instillation, all NC were phagocytosed by alveolar macrophages. Bronchoalveolar lavage fluid (BALF) and histopathological analyses revealed that CNF2 induced limited inflammation with granuloma formation and minimal BALF changes, whereas CNF1 and CNC1 triggered similar changes in BALF inflammatory markers. Although CNC1 elicited the most notable histopathological changes among NCs, all NC-induced responses were less severe than those caused by MWCNTs. No significant alterations were observed in lymphocyte subsets in the spleen or thymus, indicating minimal systemic immunotoxicity. In vitro assays using NR8383 alveolar macrophages were performed to compare CNC1, sulfuric acid-hydrolyzed CNC2, and desulfurized CNC3. All CNCs were internalized and stimulated pro-inflammatory cytokine production, with responses influenced by surface chemistry despite similar size and morphology. CNC1 and CNC2 exhibited low cytotoxicity following 48h exposure at concentrations up to 100 μg/mL. In contrast, CNC3 induced mild to moderate cytotoxicity under the same conditions, upregulated genes linked to inflammatory responses, oxidative stress, apoptosis, and extracellular matrix degradation. These findings reveal that NCs generally exhibit lower pulmonary toxicity than MWCNTs; however, their biological effects are strongly modulated by fiber morphology, surface characteristics, and deposition behavior. To ensure safe use of NCs, comprehensive, material-specific toxicity assessments and standardized evaluation frameworks are essential. In particular, rigorous endotoxin testing and control should be incorporated into future studies to maintain validity and reproducibility of hazard evaluations. Additionally, long-term and repeated exposure models, mechanistic investigations, and case-by-case safety assessments are required, especially for NC variants with limited biodegradability and prolonged pulmonary retention.
纳米纤维素诱导的肺部炎症和免疫反应:来自体内和体外模型的见解
纳米纤维素(NC),包括纤维素纳米原纤维(CNFs)和纤维素纳米晶体(cnc),在工业、生物医学和消费品中的使用日益增加,引起了人们对潜在吸入暴露的担忧,因为这些材料含有可吸入粒径范围内的成分(<10 μ m)。尽管应用越来越广泛,但nc诱导的肺部和全身免疫效应的数据仍然有限。本研究以多壁碳纳米管(MWCNTs)为基准,研究了气管内灌注CNF1 (tempo氧化)、CNF2(机械纤颤)和CNC1对大鼠的肺和免疫毒性作用。所有材料给药剂量为2.0 mg/kg体重,纤维直径为14.1 ~ 28.2 nm,长度为0.7 ~ 2.2µm。注射后28 d,所有NC均被肺泡巨噬细胞吞噬。支气管肺泡灌洗液(BALF)和组织病理学分析显示,CNF2诱导了有限的炎症,伴有肉芽肿形成和微小的BALF变化,而CNF1和CNC1引发了类似的BALF炎症标志物变化。尽管CNC1在nc中引起了最显著的组织病理学变化,但所有nc诱导的反应都不如MWCNTs引起的反应严重。脾脏或胸腺淋巴细胞亚群未见明显改变,表明全身免疫毒性很小。采用NR8383肺泡巨噬细胞进行体外实验,比较CNC1、硫酸水解的CNC2和脱硫的CNC3。所有的cnc都被内化并刺激促炎细胞因子的产生,尽管大小和形态相似,但其反应受表面化学的影响。CNC1和CNC2在浓度高达100 μg/mL的条件下暴露48小时后表现出较低的细胞毒性。相反,在相同条件下,CNC3诱导轻度至中度细胞毒性,上调与炎症反应、氧化应激、细胞凋亡和细胞外基质降解相关的基因。这些发现表明,NCs通常比MWCNTs表现出更低的肺毒性;然而,它们的生物效应受到纤维形态、表面特性和沉积行为的强烈调节。为了确保NCs的安全使用,全面的、特定材料的毒性评估和标准化评价框架至关重要。特别是,严格的内毒素检测和控制应纳入未来的研究,以保持危害评价的有效性和可重复性。此外,需要长期和重复暴露模型、机制调查和逐个安全性评估,特别是对于生物降解性有限和肺潴留延长的NC变异。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current Research in Toxicology
Environmental Science-Health, Toxicology and Mutagenesis
CiteScore
4.70
自引率
3.00%
发文量
33
审稿时长
82 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


