Tuning the balance between CO2 absorption capacity and kinetics in diol-based low transition temperature mixtures: The dual role of 1,2-hexanediol
IF 5.2
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
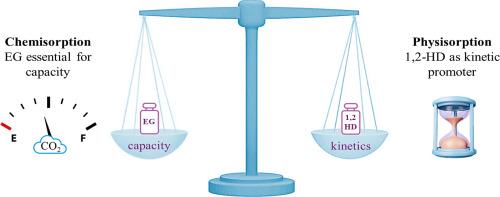
The ability of low transition temperature mixtures (LTTMs) composed of potassium hydroxide, boric acid, 1,2-diols of varying chain lengths, and water to serve as environmentally friendly solvents for CO2 capture was systematically investigated. CO2 solubility was measured at 35 and 50 °C under pressures ranging from 1 to 4 MPa. Among the tested systems, the ethylene glycol (EG)-based LTTM exhibited the highest CO2 absorption capacity, while the 1,2-hexanediol (1,2-HD)-based LTTM showed the fastest absorption kinetics, despite a lower overall uptake. Blends of EG or 1,2-propanediol (1,2-PD) with 1,2-HD demonstrated a synergistic kinetic enhancement, accelerating CO2 uptake compared with single-diol systems. This kinetic advantage came at the expense of reduced absorption capacity relative to the parent EG- and 1,2-PD-based LTTMs, although total uptake remained higher than that of the 1,2-HD system. Notably, in the 1,2-HD-based LTTM, a shift in the CO2 absorption mechanism from chemical to physical was observed, indicating its role as a kinetic rather than thermodynamic promoter. Preliminary computational studies support this observation, suggesting that the butyl chains of 1,2-HD molecules may (i) shield the OH groups, hindering CO2 chemical capture, and (ii) form non-polar pockets capable of hosting CO2 molecules, favouring their physical absorption. These findings emphasise the importance of short-chain diols in maintaining adequate absorption capacity.
Overall, this work highlights the potential of compositional tuning in LTTMs to optimise kinetic and thermodynamic performance in CO2 capture, offering valuable insights for the rational design of green, high-performance sorbents tailored to specific process conditions.
调整以二醇为基础的低转变温度混合物中二氧化碳吸收能力和动力学之间的平衡:1,2-己二醇的双重作用
系统研究了氢氧化钾、硼酸、不同链长的1,2-二醇和水组成的低温混合物(LTTMs)作为环境友好型CO2捕集溶剂的能力。在35°C和50°C下,在1至4 MPa的压力范围内测量CO2的溶解度。在测试的体系中,以乙二醇(EG)为基础的LTTM表现出最高的CO2吸收能力,而以1,2-己二醇(1,2- hd)为基础的LTTM表现出最快的吸收动力学,尽管总体吸收率较低。与单一的二醇体系相比,EG或1,2-丙二醇(1,2- pd)与1,2- hd的共混体系表现出协同动力学增强,加速了二氧化碳的吸收。这种动力学优势是以相对于母体EG-和1,2- pd - LTTMs的吸收能力降低为代价的,尽管总吸收量仍然高于1,2- hd体系。值得注意的是,在基于1,2- hd的LTTM中,观察到CO2吸收机制从化学到物理的转变,表明其作为动力学而不是热力学促进剂的作用。初步的计算研究支持这一观察结果,表明1,2- hd分子的丁基链可能(i)屏蔽OH基团,阻碍CO2的化学捕获,以及(ii)形成能够容纳CO2分子的非极性口袋,有利于它们的物理吸收。这些发现强调了短链二醇在保持足够吸收能力方面的重要性。总的来说,这项工作强调了ltm中成分调整的潜力,以优化二氧化碳捕获中的动力学和热力学性能,为合理设计适合特定工艺条件的绿色高性能吸附剂提供了有价值的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


