Solvation of alkali metal ions in liquid hydrogen fluoride and water: A combined ab initio and molecular dynamics study
IF 5.2
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
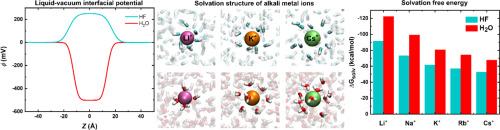
Despite its high toxicity and corrosivity, hydrogen fluoride (HF) is widely used in industrial processes such as fluorine compound synthesis, aluminum production, and gasoline refining. As the only weak hydrohalic acid, HF may exist in its molecular, undissociated form in some aqueous and biological environments. HF has similar polarity to H₂O, and both liquids exhibit hydrogen bond-driven molecular associations. However, their electrostatic potential surfaces differ: the oxygen atom in H₂O carries a more negative potential than the fluorine atom in HF, while the hydrogen atom in HF carries a more positive potential than in H₂O. Although many salts dissolve in HF, their solvation properties remain poorly understood. Here, we present the first computational investigation of alkali metal ion (Li+, Na+, K+, Rb+, Cs+) solvation in liquid HF. High-level ab initio calculations on (HF)ₙM+ clusters (n = 1–6) show gas-phase binding affinities 30–35 % lower than for water. Our results reveal that ion–dipole and hydrogen bonding interactions can act cooperatively or anti-cooperatively depending on cluster geometry, with cooperative effects generally dominant. Molecular dynamics simulations using a non-polarizable model yield average HF coordination numbers of 5.5, 8.1, 8.9, and 9.7 for Li+ to Cs+, compared to 4.2, 5.9, 7.1, 8.1, and 9.6 in water. The computed liquid–vacuum interfacial potential is +250 mV for HF and −500 mV for H₂O. The calculated solvation free energies are −91.5 to −52.6 kcal/mol in HF versus −122.4 to −67.6 kcal/mol in water. These results show that solvation is more favorable in water, consistent with higher ion–H₂O affinities and more negative interfacial potential of water.
碱金属离子在液氟氢和水中的溶剂化:从头算和分子动力学的结合研究
尽管氟化氢具有高毒性和腐蚀性,但它被广泛应用于氟化合物合成、铝生产和汽油精炼等工业过程中。氟化氢作为唯一的弱氢盐酸,在某些水环境和生物环境中可能以分子未解离形式存在。HF与h2o具有相似的极性,两种液体均表现出氢键驱动的分子结合。然而,它们的静电电位表面不同:h2o中的氧原子比HF中的氟原子携带更多的负电位,而HF中的氢原子比h2o中携带更多的正电位。虽然许多盐溶解在HF中,但人们对它们的溶剂化性质仍然知之甚少。本文首次对碱金属离子(Li+, Na+, K+, Rb+, Cs+)在液态HF中的溶剂化进行了计算研究。对(HF) M+簇(n = 1-6)的高水平从头计算表明,气相结合亲和度比水低30 - 35%。我们的研究结果表明,离子偶极子和氢键相互作用可以协同或反协同作用,这取决于簇的几何形状,通常以协同效应为主。采用非极化模型进行分子动力学模拟,Li+与Cs+的平均HF配位数分别为5.5、8.1、8.9和9.7,而水中的平均HF配位数分别为4.2、5.9、7.1、8.1和9.6。计算得到的液-真空界面电位HF为+ 250mv, h2o为- 500mv。计算得到HF的溶剂化自由能为- 91.5 ~ - 52.6 kcal/mol,而水的溶剂化自由能为- 122.4 ~ - 67.6 kcal/mol。这些结果表明,在水中溶剂化更有利,这与较高的离子- h₂O亲和和更高的负界面势相一致。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Liquids
化学-物理:原子、分子和化学物理
CiteScore
10.30
自引率
16.70%
发文量
2597
审稿时长
78 days
期刊介绍:
The journal includes papers in the following areas:
– Simple organic liquids and mixtures
– Ionic liquids
– Surfactant solutions (including micelles and vesicles) and liquid interfaces
– Colloidal solutions and nanoparticles
– Thermotropic and lyotropic liquid crystals
– Ferrofluids
– Water, aqueous solutions and other hydrogen-bonded liquids
– Lubricants, polymer solutions and melts
– Molten metals and salts
– Phase transitions and critical phenomena in liquids and confined fluids
– Self assembly in complex liquids.– Biomolecules in solution
The emphasis is on the molecular (or microscopic) understanding of particular liquids or liquid systems, especially concerning structure, dynamics and intermolecular forces. The experimental techniques used may include:
– Conventional spectroscopy (mid-IR and far-IR, Raman, NMR, etc.)
– Non-linear optics and time resolved spectroscopy (psec, fsec, asec, ISRS, etc.)
– Light scattering (Rayleigh, Brillouin, PCS, etc.)
– Dielectric relaxation
– X-ray and neutron scattering and diffraction.
Experimental studies, computer simulations (MD or MC) and analytical theory will be considered for publication; papers just reporting experimental results that do not contribute to the understanding of the fundamentals of molecular and ionic liquids will not be accepted. Only papers of a non-routine nature and advancing the field will be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


