Dynamics of Volatile Products in the Electro-oxidation of Ethanol on Pt/C and PtSn/C Catalysts
IF 3.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Ethanol electro-oxidation reaction (EEOR) is a strategically important reaction for the development of fuel cells and low-temperature electrolysis technologies, although its application is hindered by slow kinetics and poor selectivity toward the C1 pathway (CO2 formation) on platinum-based catalysts. In this study, the dynamic formation of volatile products (acetaldehyde, acetic acid, and CO2) was investigated during the EEOR on Pt/C and PtSn/C electrodes in acidic media, with particular emphasis on oscillatory conditions using online electrochemical mass spectrometry (OLEMS). PtSn/C catalysts were synthesized via chemical reduction and characterized by XRD, TEM, and XPS, revealing smaller particle sizes, a higher proportion of oxidized Pt species, and the presence of Sn4+ as SnO2. OLEMS experiments showed earlier onset for acetaldehyde, CO2, and acetic acid products for PtSn/C. Despite the low volatility of acetic acid, its production was confirmed and quantified by high-performance liquid chromatography (HPLC), reaching up to 965.2 ppm at 0.5 V, which is 97 times higher than that of Pt/C. Multivariate linear regression (MLR) analysis revealed a greater contribution of acetic acid and CO2 to the faradaic current for PtSn/C, indicating a substantial shift in the reaction pathway. Under oscillatory conditions, PtSn/C exhibited greater robustness, lower potential amplitude, and reduced susceptibility to surface poisoning. These results highlight the role of Sn in enhancing surface regeneration and promoting deeper oxidation pathways, demonstrating the importance of catalyst design in tuning the selectivity and efficiency of the EEOR.
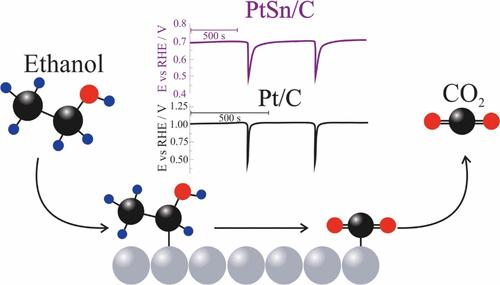
Pt/C和PtSn/C催化剂上乙醇电氧化挥发性产物的动力学研究
乙醇电氧化反应(EEOR)对燃料电池和低温电解技术的发展具有重要的战略意义,尽管其在铂基催化剂上对C1途径(CO2生成)的缓慢动力学和低选择性阻碍了其应用。在这项研究中,研究了酸性介质中Pt/C和PtSn/C电极上EEOR过程中挥发性产物(乙醛、乙酸和二氧化碳)的动态形成,特别强调了使用在线电化学质谱(OLEMS)的振荡条件。采用化学还原法制备了PtSn/C催化剂,并通过XRD、TEM和XPS对其进行了表征,结果表明,该催化剂粒径更小,氧化Pt组分比例更高,Sn4+以SnO2形式存在。OLEMS实验显示,PtSn/C的乙醛、CO2和乙酸产物发病较早。尽管乙酸的挥发性很低,但通过高效液相色谱(HPLC)证实了其产量并进行了定量,在0.5 V下可达965.2 ppm,比Pt/C高97倍。多元线性回归(MLR)分析显示,醋酸和CO2对PtSn/C的法拉第电流的贡献更大,表明反应途径发生了实质性的转变。在振荡条件下,PtSn/C表现出更强的鲁棒性、更低的电位振幅和更低的表面中毒敏感性。这些结果突出了Sn在增强表面再生和促进深层氧化途径中的作用,证明了催化剂设计在调节EEOR的选择性和效率方面的重要性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

The Journal of Physical Chemistry C
化学-材料科学:综合
CiteScore
6.50
自引率
8.10%
发文量
2047
审稿时长
1.8 months
期刊介绍:
The Journal of Physical Chemistry A/B/C is devoted to reporting new and original experimental and theoretical basic research of interest to physical chemists, biophysical chemists, and chemical physicists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


