NiH-Catalyzed Homobenzylic Hydroalkylation of Aryl Alkenes Using Sulfoxonium Ylides
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
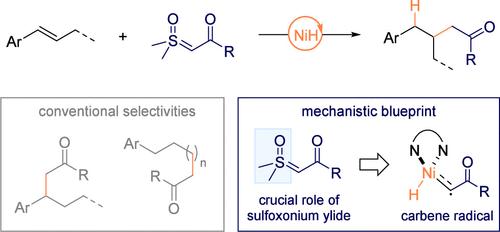
We present here the first example of NiH-catalyzed homobenzylic hydroalkylation of aryl alkenes mediated by a nickel carbene radical, achieving the elusive β-selectivity with excellent regiocontrol. Mechanistic investigations suggest that this transformation is enabled by the preferential engagement of NiH with bench-stable sulfoxonium ylides, whose unique chelation properties promote carbene activation prior to alkene insertion. The resulting nickel carbene radical is proposed to undergo selective β-addition, followed by intramolecular metal hydride transfer and protodemetalation. The reaction exhibits broad scope across aryl, heteroaryl, and complex bioactive alkene derivatives, as well as diverse sulfoxonium ylides. This work establishes a new mechanistic platform for NiH catalysis, expanding the synthetic repertoire for site-selective alkene functionalization.
用亚砜鎓化物催化芳基烯烃的同双酶烷基化反应
我们在这里提出了第一个由镍碳自由基介导的nih催化芳基烯烃的同型酶烷基化的例子,实现了难以捉摸的β-选择性和良好的区域控制。机制研究表明,这种转化是由NiH优先参与稳定的亚砜鎓化物,其独特的螯合特性促进了烯烃插入之前的碳活化。得到的镍羰基经过选择性β加成,然后是分子内金属氢化物转移和原金属脱金属作用。该反应范围广泛,包括芳基、杂芳基和复杂的生物活性烯烃衍生物,以及各种亚砜酰化物。这项工作建立了一个新的NiH催化机制平台,扩大了选择性烯烃功能化的合成曲目。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


