Regiodivergent Access to α- and β-Amino Acids via Solvent-Controlled Rh-Catalyzed Carboamidation of β,γ-Unsaturated Carboxylic Acids
IF 15.6
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Non-natural α- and β-amino acids are pivotal building blocks in medicinal chemistry and bioactive molecule discovery. Herein, we report a rhodium-catalyzed, solvent-directed, regiodivergent carboamidation of β,γ-unsaturated alkenyl carboxylic acid for the programmable synthesis of both α- and β-amino acids. This strategy leverages β,γ-unsaturated alkenyl carboxylic acids, organoboronic acids, and dioxazolones as readily available substrates, achieving broad substrate scope with high efficiency (average yield 60%) and excellent functional group tolerance. The solvent system (HFIP vs THF) serves as a simple switch to precisely control regioselectivity (α:β up to >20:1 or β:α up to >20:1), bypassing traditional protecting-group manipulations. Notably, this protocol enables concise two-step synthesis of N-Ac sitagliptin and rapid access to diverse peptidomimetics, showcasing its potential in streamlining the development of pharmaceuticals and bioactive probes. DFT calculations reveal distinct energy barriers for the rate-determining steps of the two pathways in HFIP and THF, rationalizing the solvent-controlled selectivity.
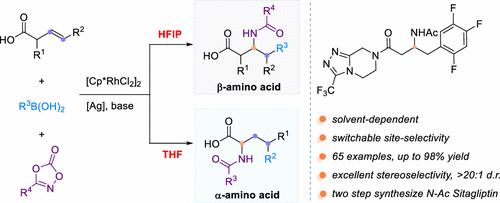
通过溶剂控制的铑催化β,γ-不饱和羧酸的碳酰胺化获得α-和β-氨基酸的区域发散性
非天然α-和β-氨基酸是药物化学和生物活性分子发现的关键组成部分。在此,我们报道了一种铑催化、溶剂导向、区域发散的β,γ-不饱和烯基羧酸的碳酰胺化反应,用于可编程合成α-和β-氨基酸。该策略利用β,γ-不饱和烯基羧酸,有机硼酸和二恶唑酮作为现成的底物,实现了底物范围广,效率高(平均收率60%)和优异的官能团耐受性。溶剂系统(HFIP vs THF)作为一个简单的开关,以精确控制区域选择性(α:β高达>;20:1或β:α高达>;20:1),绕过传统的保护基团操作。值得注意的是,该方案能够简洁地两步合成N-Ac西格列汀,并快速获得各种肽模拟物,显示其在简化药物和生物活性探针开发方面的潜力。DFT计算揭示了HFIP和THF两种途径的速率决定步骤的不同能量势垒,使溶剂控制的选择性合理化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
24.40
自引率
6.00%
发文量
2398
审稿时长
1.6 months
期刊介绍:
The flagship journal of the American Chemical Society, known as the Journal of the American Chemical Society (JACS), has been a prestigious publication since its establishment in 1879. It holds a preeminent position in the field of chemistry and related interdisciplinary sciences. JACS is committed to disseminating cutting-edge research papers, covering a wide range of topics, and encompasses approximately 19,000 pages of Articles, Communications, and Perspectives annually. With a weekly publication frequency, JACS plays a vital role in advancing the field of chemistry by providing essential research.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


