Early-life ketone body signalling promotes beige fat biogenesis through changes in histone acetylome and β-hydroxybutyrylome
IF 20.8
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Infants undergo distinct ketogenesis during the preweaning period, yet its physiological implications remain unclear. Here, we show that preweaning ketosis promotes beige fat biogenesis and improves health outcomes in adulthood. Loss of ketogenesis in neonatal mice by early weaning or ablation of Hmgcs2 hinders beige adipogenesis, subsequently exacerbating metabolic dysregulation in high-fat diet-induced obesity. Enhanced ketogenesis during lactation through exogenous ketone supplements enhances energy expenditure, beige fat formation, and mitochondrial biogenesis and respiration. Using single-cell RNA sequencing, we identified a subset of β-hydroxybutyrate-responsive adipocyte progenitor cells (APCs) expressing Cd81 that showed high beige adipogenic potential. Enhanced ketogenesis promotes the recruitment of beige APCs and their differentiation into beige adipocytes. Mechanistically, ketogenesis-derived βHB induces a switch in the histone acetylome and β-hydroxybutyrylome for transcriptional activation of beige fat biogenesis genes. Notably, enhanced ketogenesis during lactation alleviates adverse metabolic effects predisposed by parental obesity. Our study highlights that targeting preweaning ketosis to drive beige adipogenesis may offer a therapeutic approach to combat obesity and metabolic diseases in adulthood. In the context of parental or diet-induced obesity, preweaning ketosis contributes to improved health outcomes, particularly by regulating the histone acetylome and β-hydroxybutyrylome for transcriptional activation of beige fat biogenesis genes.
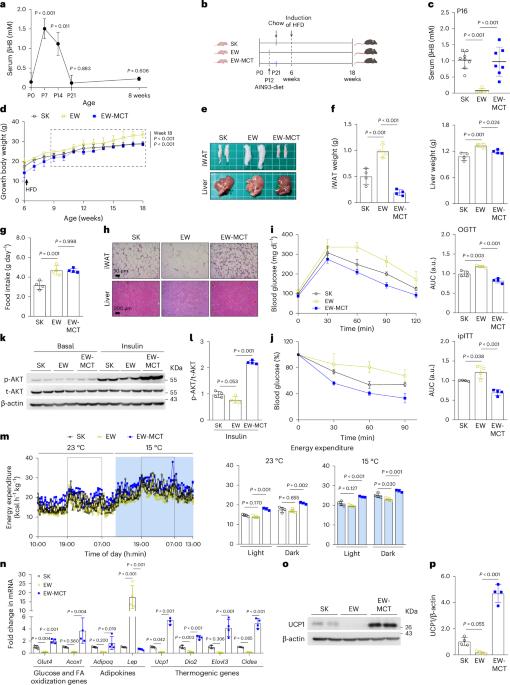
早期生活酮体信号通过组蛋白乙酰基和β-羟基丁基的变化促进米色脂肪的生物生成。
婴儿在断奶前经历不同的生酮过程,但其生理意义尚不清楚。本研究表明,断奶前酮症可促进米色脂肪的生物生成,并改善成年后的健康状况。新生小鼠早期断奶或Hmgcs2消融导致的生酮功能丧失阻碍了米色脂肪的形成,随后加剧了高脂肪饮食引起的肥胖的代谢失调。在哺乳期通过外源性酮补充增强酮生成,增加能量消耗,米色脂肪形成,线粒体生物发生和呼吸。通过单细胞RNA测序,我们发现了一个表达Cd81的β-羟基丁酸反应性脂肪细胞(APCs)亚群,显示出高米色脂肪生成潜力。增强的生酮促进了米色apc的募集和向米色脂肪细胞的分化。机制上,酮生衍生的βHB诱导组蛋白乙酰基和β-羟基丁基体的开关,以转录激活米色脂肪生物发生基因。值得注意的是,哺乳期间增强的生酮减轻了父母肥胖造成的不良代谢影响。我们的研究强调,以断奶前酮症为目标来驱动米色脂肪生成可能为对抗成年期肥胖和代谢疾病提供一种治疗方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


