Developing SEMA4A-directed CAR T cells to overcome low BCMA antigen density in multiple myeloma
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
Chimeric antigen receptor (CAR) T cell therapy targeting B cell maturation antigen (BCMA) for multiple myeloma (MM) is effective, but relapses associated with low-to-negative BCMA expression are common, indicating the need for additional targets. We quantitatively profile antigen density in a cohort of patients relapsed after BCMA CAR T therapy, showing high number of SEMA4A molecules/cell where BCMA density is low. SEMA4A deletion limits MM cell growth, migration, tissue infiltration, and osteoclast formation, while extending mouse survival. We generate monoclonal antibodies targeting SEMA4A-extracellular domain for CAR construction, screen engineered T cells for expansion, cytokine release, and cytotoxicity against MM cells. Lead constructs lack reactivity against normal non-hematopoietic tissues. SEMA4A CAR T cells show superior efficacy than BCMA CAR T cells eliminating patient-derived BCMAlow tumors and MM cells progressing under suboptimal doses of BCMA CAR T cells. This study prepares for a phase 1 clinical trial with SEMA4A-directed CAR T cells for MM.
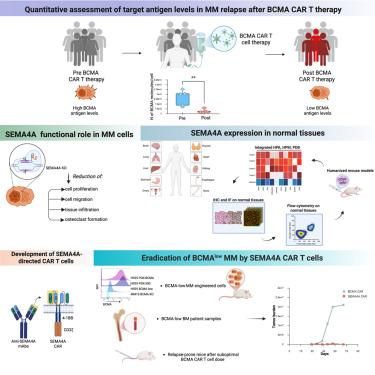
开发sema4a定向CAR - T细胞克服多发性骨髓瘤低BCMA抗原密度
靶向B细胞成熟抗原(BCMA)的嵌合抗原受体(CAR) T细胞治疗多发性骨髓瘤(MM)是有效的,但与BCMA低至阴性表达相关的复发是常见的,这表明需要额外的靶点。我们定量分析了一组BCMA CAR - T治疗后复发患者的抗原密度,显示在BCMA密度低的地方,SEMA4A分子/细胞数量高。SEMA4A缺失限制了MM细胞的生长、迁移、组织浸润和破骨细胞的形成,同时延长了小鼠的存活时间。我们产生靶向sema4a细胞外结构域的单克隆抗体,用于CAR构建,筛选工程T细胞的扩增,细胞因子释放和对MM细胞的细胞毒性。铅构建体对正常非造血组织缺乏反应性。SEMA4A CAR - T细胞在次优剂量的BCMA CAR - T细胞下,对患者源性BCMAlow肿瘤和MM细胞的清除效果优于BCMA CAR - T细胞。该研究准备用sema4a靶向CAR - T细胞治疗MM的1期临床试验。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


