Neoadjuvant nab-paclitaxel plus gemcitabine followed by modified FOLFIRINOX for resectable pancreatic cancer: A randomized phase 3 trial
IF 44.5
1区 医学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
This single-center, randomized phase 3 trial (NCT03750669) evaluated sequential neoadjuvant nab-paclitaxel plus gemcitabine followed by modified FOLFIRINOX versus upfront surgery in 324 patients with resectable pancreatic cancer. Patients in the neoadjuvant group received nab-paclitaxel plus gemcitabine followed by modified FOLFIRINOX before surgery and then four cycles of adjuvant therapy (preferably gemcitabine plus capecitabine), while those in the upfront surgery group underwent immediate resection followed by six cycles of adjuvant therapy. The primary endpoint was event-free survival. Notably, 50% of patients had tumors in the pancreatic body or tail. Median event-free survival was 15.3 months (95% confidence interval [CI], 12.6–19.3) versus 10.9 months (95% CI, 9.1–13.5; hazard ratio [HR], 0.71; 95% CI, 0.54–0.93; p = 0.0136). Median overall survival was 35.4 months versus 27.2 months (HR, 0.73; 95% CI, 0.53–1.00; nominal p = 0.0477). Grade ≥3 adverse events occurred in 47.6% versus 30.7% of patients. This neoadjuvant regimen improves event-free survival with manageable safety.
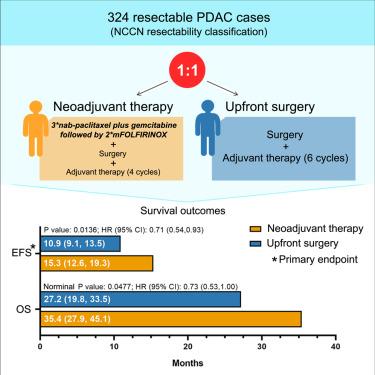
新辅助nab-紫杉醇加吉西他滨后改良FOLFIRINOX治疗可切除胰腺癌:一项随机3期试验
这项单中心、随机3期试验(NCT03750669)评估了324例可切除胰腺癌患者的序贯新辅助nab-紫杉醇加吉西他滨后改良FOLFIRINOX与前期手术的对比。新辅助组患者术前接受nab-紫杉醇+吉西他滨,随后改良FOLFIRINOX,然后进行4个周期的辅助治疗(最好是吉西他滨+卡培他滨),而术前组患者接受立即切除,然后进行6个周期的辅助治疗。主要终点为无事件生存期。值得注意的是,50%的患者在胰腺体或胰腺尾有肿瘤。中位无事件生存期为15.3个月(95%可信区间[CI], 12.6-19.3) vs 10.9个月(95% CI, 9.1-13.5;风险比[HR], 0.71; 95% CI, 0.54-0.93; p = 0.0136)。中位总生存期为35.4个月对27.2个月(HR, 0.73; 95% CI, 0.53-1.00;名义p = 0.0477)。≥3级不良事件发生率分别为47.6%和30.7%。这种新辅助方案提高了无事件生存和可控的安全性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cancer Cell
医学-肿瘤学
CiteScore
55.20
自引率
1.20%
发文量
179
审稿时长
4-8 weeks
期刊介绍:
Cancer Cell is a journal that focuses on promoting major advances in cancer research and oncology. The primary criteria for considering manuscripts are as follows:
Major advances: Manuscripts should provide significant advancements in answering important questions related to naturally occurring cancers.
Translational research: The journal welcomes translational research, which involves the application of basic scientific findings to human health and clinical practice.
Clinical investigations: Cancer Cell is interested in publishing clinical investigations that contribute to establishing new paradigms in the treatment, diagnosis, or prevention of cancers.
Insights into cancer biology: The journal values clinical investigations that provide important insights into cancer biology beyond what has been revealed by preclinical studies.
Mechanism-based proof-of-principle studies: Cancer Cell encourages the publication of mechanism-based proof-of-principle clinical studies, which demonstrate the feasibility of a specific therapeutic approach or diagnostic test.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


